Title: The Tell-Tale Heart(s), Part 2: Donor-Derived Invasive Fungal Infections among Heart Transplant Recipients
Submitted by: Jay Krishnan, John Dougherty, Joseph Barwatt, Manuela Carugati, Rachel Miller, Cameron Wolfe, John Perfect, Julia Messina
Institution: Duke University
Email: jrk26@duke.edu
History:
A 59-year-old man was admitted for orthotopic heart transplantation. His past medical history was notable for non-ischemic cardiomyopathy status post implantable cardiac defibrillator (ICD) and prior sternotomy for implantation of a left ventricular assist device (LVAD).
Donor history: One month prior to his death, the 32-year-old male organ donor presented to his primary care physician with weight loss and decompensated cirrhosis. He had a profound leukocytosis to 40,000 cells/microliter at that time but did not undergo further evaluation. Subsequently, he suffered a motor vehicle accident, was admitted to the hospital, and progressed to brain death due to multifocal intracranial hemorrhage, which had a radiographic distribution that could have been consistent with an embolic phenomenon. At the time of hospital presentation after the motor vehicle accident, profound leukocytosis was notable (40,900 cells/microliter). Pre-transplantation work-up of the donor included routine blood cultures and echocardiography, which were unremarkable.
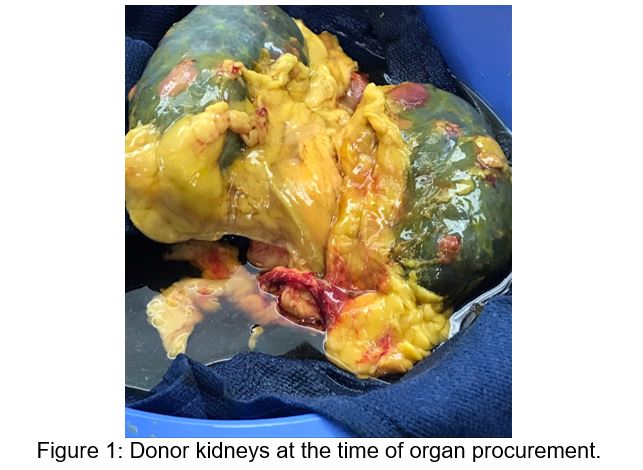
During the transplant surgery, the organ recipient’s ICD and LVAD were removed successfully. The donor heart was inspected with normal appearance of the aortic, mitral, and tricuspid valves. After the procedure, the transplant surgery team was notified that the gross pathology from donor kidneys and adrenal glands returned with micro-abscesses (Figure 1). Post-operatively, the transplant recipient received empiric vancomycin and piperacillin-tazobactam along with routine antimicrobial prophylaxis based on institutional post-transplantation protocols.
Further preliminary histopathology review of the donor’s left and right kidneys demonstrated hyaline hyphal forms. The recipient was started on micafungin 150mg intravenous (IV) daily pending further details from the donor’s renal tissue histopathology and cultures.
Physical Examination:
Maximum temperature of 38.4 Celsius over the 24 hours on post-operative day 1, heart rate 100/min, blood pressure 116/77 mm Hg on dopamine, respiratory rate 22, oxygen saturation 100% on PC/AC with 40% FIO2 and PEEP 8
General: Intubated and sedated. Appeared stated age
HEENT: PERRL. No conjunctival injection. Oropharynx poorly visualized due to endotracheal tube but no overt lesions. Moist mucous membranes
Cardiovascular: Regular rhythm. Pericardial rub present. No murmurs or gallops.
Chest: Surgical bandage in place. Four anterior chest tubes draining serosanguinous fluid.
Respiratory: Mechanical breath sounds. Clear to auscultation with good air exchange
Abdomen: Soft, nontender, nondistended, normoactive bowel sounds
Extremities: 1+ pedal edema. Warm and well-perfused
Skin: No skin lesions observed on upper extremities, lower extremities, or trunk. Central lines without surrounding erythema, exudate, or induration
Laboratory Examination:
WBC: 19,100 cells/microliter, Hemoglobin: 10.2 g/dL, Platelet: 232/mL, Creatinine 1.4 (baseline 0.9) mg/dL, AST 126 U/L, ALT 33 U/L, Alkaline Phosphatase 38 U/L, Total Bilirubin 2.5 mg/dL.
Radiology:
Post-operative transesophageal echocardiography (TEE) demonstrated normal left ventricular function without valvular abnormalities of note.
Question 1: What are probable/possible diagnoses?
Based on preliminary donor histopathology, a disseminated mold infection was suspected. The differential diagnosis included infection with the Mucorales genera, or, if septate hyphae were present, Aspergillus species and Fusarium species are possibilities, among others.
Microbiology/Diagnostic Tests Performed:
Histopathology and cultures from donor kidney and adrenal samples confirmed Aspergillus fumigatus.
Initial course:
Despite the concerning donor tissue findings, the recipient was clinically improving without evidence of invasive fungal infection clinically or radiographically. Isavuconazonium therapy (372mg every 8 hours for six doses followed by 372mg daily) was initiated in the recipient as a precautionary measure, and micafungin therapy was discontinued shortly thereafter. The recipient was extubated and weaned from inotropes on post-operative day 4; his incision site after surgical bandage removal on post-operative day 6 appeared healthy. He had no further fevers with a stable leukocytosis around 13,000/mL.
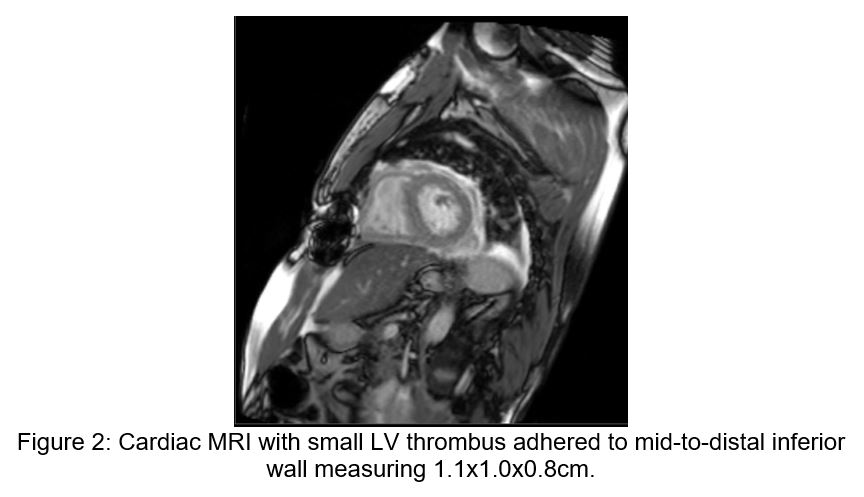
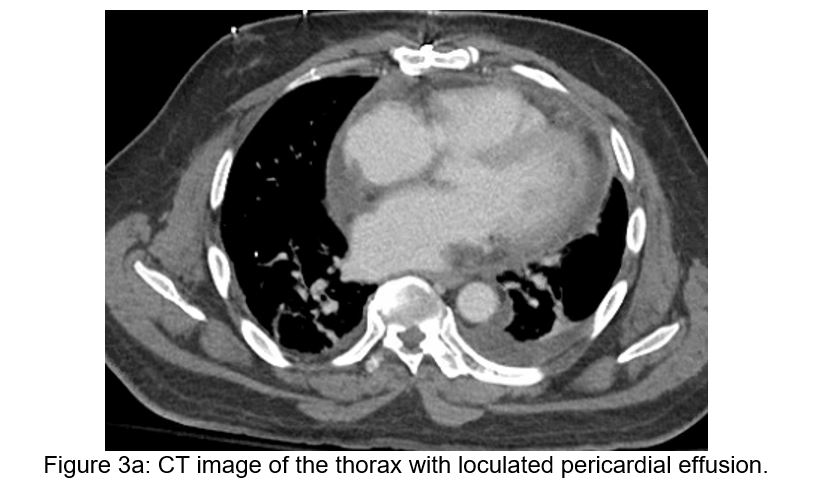
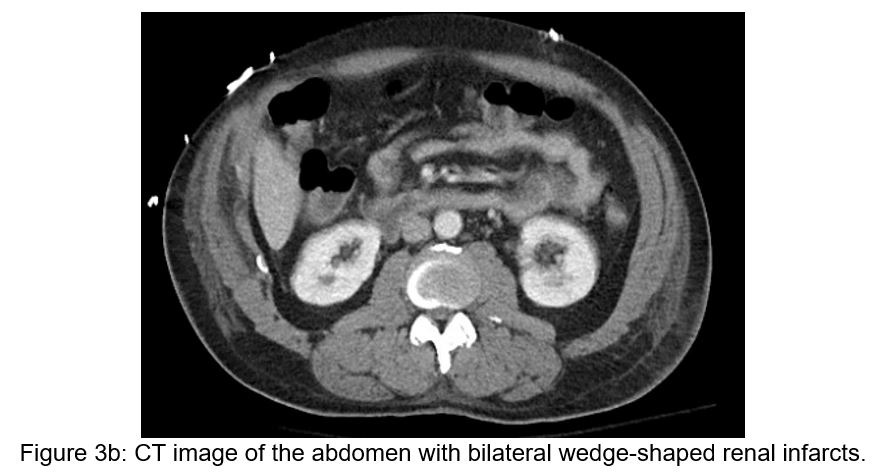
On post-operative day 7, the recipient then developed mild hypoactive delirium without focal neurologic deficits, followed by a mounting leukocytosis. A non-contrasted head CT scan demonstrated a small acute left corpus striatum infarct, and subsequent brain MRI showed numerous acute infarcts throughout the bilateral hemispheres concerning for embolic phenomena. A post-operative cardiac MRI obtained concurrently demonstrated a left ventricular thrombus along the inferior septal wall, though ventricular and valvular morphology and function were unremarkable (see Figure 2). Transesophageal echocardiogram confirmed normal valvular morphology as well as a mobile mass on the mid-distal inferoseptal wall consistent with thrombus. Lastly, a CT scan of the chest, abdomen, and pelvis demonstrated a small, partially loculated pericardial effusion, a developed retrosternal fluid collection, and bilateral renal wedge-shaped hypodensities concerning for multifocal infarction (Figure 3a and 3b).
Serum galactomannan antigen testing was positive at 0.568 index units (Mayo Laboratories). Fungal blood cultures and later mediastinal tissue cultures from the recipient grew Aspergillus fumigatus.
Final Diagnosis:
Donor-derived Aspergillus fumigatus endovascular infection leading to fungemia, mediastinitis, and suspected infected intracardiac thrombus complicated by septic cerebrovascular emboli in a heart transplant recipient.
Question 2: What treatment is recommended in the care of this patient?
Treatment:
The isavuconazonium therapy was discontinued, and the patient was initiated on voriconazole 6mg/kg every twelve hours for two doses followed by 4mg/kg every twelve hours. Micafungin 150mg IV daily was resumed as well.
Outcome:
Initially, the patient’s mental status improved on the above therapy. Unfortunately, five days on the revised regimen, he developed a new right gaze preference and left-sided hemiparesis. CT imaging revealed a large right intraparenchymal hemorrhage complicated by partial herniation requiring neurosurgical evacuation.
He underwent seven sternal debridement procedures to obtain mediastinal source control. Mediastinal tissue cultures collected with each debridement over 7 days grew Aspergillus fumigatus, but by the eighth day, these tissue cultures returned negative and his chest was closed. He continued on voriconazole and micafungin for approximately 6 weeks, at which time he developed a significant liver injury that prompted a switch to posaconazole and micafungin.
Over his 11-month hospitalization, his course was complicated by line-associated bloodstream infections, C. difficile infection, acute cholecystitis, seizures, and COVID-19 infection. His neurologic functional status has slowly improved, with progressive resolution of his aphasia but persistent partial left-sided hemiparesis.
Discussion: (500 words):
Here we describe a highly unusual case of confirmed donor-derived invasive fungal infection (IFI) due to Aspergillus fumigatus in the early post-operative period after heart transplantation. The organ donor presumably had sub-radiographic Aspergillus endocarditis prior to transplantation, as evidenced by signs of metastatic infection including bilateral renal abscesses and suspected embolic cerebral hemorrhagic infarcts. This endovascular infection was subsequently transmitted to the recipient, who developed mediastinitis and an infected intracardiac thrombus with concurrent Aspergillus fungemia. Aggressive surgical debridement and antifungal management were required to clear the recipient’s blood and mediastinal cultures. Unfortunately, the patient suffered substantial morbidity from this infection, including an intraparenchymal cerebral hemorrhage with dense hemiparesis.
Donor-derived invasive fungal infections (IFIs) among solid organ transplant recipients are rare occurrences that carry significant morbidity and mortality. A 2020 report from the Organ Procurement and Transplantation Network found that unexpected donor-derived fungal infections affected 1-2 out of every 10,000 solid organ transplant recipients between 2008 and 2017 in the United States.1 Out of 2185 reports of possible donor transmission of disease, only 3 cases of confirmed donor-to-recipient transmission of Aspergillus species occurred over the ten-year study period.
Case reports of donor-derived Aspergillus IFIs among transplant recipients are correspondingly rare.One systematic review from 2013 identified only 24 cases of donor-derived IFI with filamentous fungi.2 Aspergillus species were responsible for over 70% of these cases, but the majority (>90%) in this series occurred among renal transplant recipients.
In contrast, only one additional case of confirmed donor-derived Aspergillus IFI in a heart transplant recipient has been reported since 2012.3 In that report, the organ donor was a liver transplant recipient who developed acute liver failure from graft rejection despite escalating anti-rejection therapy. He ultimately died from massive intracranial bleeding two weeks after his transplant. He was diagnosed probable invasive pulmonary aspergillosis based on lung imaging and respiratory culture growing Aspergillus fumigatus after his organs were procured. After two renal transplant recipients developed aspergillosis and underwent transplant nephrectomies in the subsequent two months, the heart transplant recipient from this organ donor would also present with Aspergillus aortic valve endocarditis and endophthalmitis six months post-transplant. Unlike our case, this patient lived without sustained morbidity though the patient required aortic valve surgery and extensive antifungal therapy.
As the organ donor pool expands to include those with higher-risk comorbidities and behaviors, we must be vigilant for subclinical infections in the organ donor. Donor-specific risk factors known to be associated with transmission of filamentous IFI to organ recipients include liver dysfunction, pre-existing immunocompromised state, prolonged ICU stay, prolonged mechanical ventilation, near-drowning experiences, and transplant tourism.2,4-5 Notably, cirrhosis (independent of transplant status) is a significant risk factor for invasive aspergillosis, with estimated rates of IFIs due to Aspergillus species approaching 2.8% among patients with cirrhosis.6 These donor risk factors should be carefully considered in the assessment of transplant recipients, particularly if the donor has unexplained clinical findings such as pulmonary nodules, leukocytosis, or central nervous system lesions.
Management of Aspergillus fumigatus endocarditis in both transplant and non-transplant populations includes antifungal therapy as well as surgical interventions with valve replacement; Aspergillus mediastinitis similarly requires both antifungal therapy and surgical debridement. Combination antifungal therapy is often employed, with voriconazole and/or liposomal amphotericin B as primary agents and echinocandins as adjunctive agents. Embolization is a common complication of Aspergillus endocarditis that is estimated to occur in 45-75% of affected patients. Mortality has been estimated to reach over 90%, with highest risk of mortality occurring early after diagnosis and among those managed with medical therapy alone.7-11 While mediastinal debridement was pursued for source control with our patient, the presence of intra-cardiac thrombus and absence of valvular abnormalities did not offer clear nor safe surgical targets for further intervention.
In summary, donor-derived IFIs among organ transplant recipients are rare events that can be associated with devastating outcomes. Evidence of fungal infection in a donor with concerning risk factors, such as fungal renal abscesses in the cirrhotic donor described in our case, should warrant meticulous evaluation for IFI in the transplant recipient. Organ recipients should be managed accordingly, which in the heart transplant recipient necessitates evaluation for and management of fungal endocarditis. Both antifungal therapy and surgical interventions tailored to the affected organ and other associated clinical manifestations are required for therapy of donor-derived IFI, but prognosis is frequently grim.
Key References:
- Kaul DR, Vece G, Blumberg E, La Hoz RM, Ison MG, Green M, Pruett T, Nalesnik MA, Tlusty SM, Wilk AR, Wolfe CR, Michaels MG. Ten years of donor-derived disease: A report of the disease transmission advisory committee. Am J Transplant. 2021 Feb;21(2):689-702. doi: 10.1111/ajt.16178. Epub 2020 Jul 25. PMID: 32627325.
- Gomez CA, Singh N. Donor-derived filamentous fungal infections in solid organ transplant recipients. Curr Opin Infect Dis. 2013 Aug;26(4):309-16. doi: 10.1097/QCO.0b013e3283630e4d.
- Keating MR, Guerrero MA, Daly RC, Walker RC, Davies SF. Transmission of invasive aspergillosis from a subclinically infected donor to three different organ transplant recipients. Chest. 1996 Apr;109(4):1119-24. doi: 10.1378/chest.109.4.1119.
- Falcone M, Massetti AP, Russo A, Vullo V, Venditti M. Invasive aspergillosis in patients with liver disease. Med Mycol. 2011 May;49(4):406-13. doi: 10.3109/13693786.2010.535030.
- Hassan EA, Abd El-Rehim AS, Hassany SM, Ahmed AO, Elsherbiny NM, Mohammed MH. Fungal infection in patients with end-stage liver disease: low frequency or low index of suspicion. Int J Infect Dis. 2014 Jun;23:69-74. doi: 10.1016/j.ijid.2013.12.014.
- Verma N, Singh S, Singh M, Chauhan A, Pradhan P, Jaiswal N, Chakrabarti A, Singh M. Global epidemiological burden of fungal infections in cirrhosis patients: A systematic review with meta-analysis. Mycoses. 2022 Mar;65(3):266-284. doi: 10.1111/myc.13387.
- Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, Heinz WJ, Jagannatha S, Koh LP, Kontoyiannis DP, Lee DG, Nucci M, Pappas PG, Slavin MA, Queiroz-Telles F, Selleslag D, Walsh TJ, Wingard JR, Maertens JA. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann Intern Med. 2015 Jan 20;162(2):81-9. doi: 10.7326/M13-2508. Erratum in: Ann Intern Med. 2015 Mar 17;162(6):463. Erratum in: Ann Intern Med. 2019 Feb 5;170(3):220.
- Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Aug 15;63(4):e1-e60. doi: 10.1093/cid/ciw326.
- Kalokhe AS, Rouphael N, El Chami MF, Workowski KA, Ganesh G, Jacob JT. Aspergillus endocarditis: a review of the literature. Int J Infect Dis. 2010 Dec;14(12):e1040-7. doi: 10.1016/j.ijid.2010.08.005.
- Jordan AM, Tatum R, Ahmad D, Patel SV, Maynes EJ, Weber MP, Moss S, Royer TL, Tchantchaleishvili V, Massey HT, Rame JE, Zurlo JJ, Aburjania N. Infective endocarditis following heart transplantation: A systematic review. Transplant Rev (Orlando). 2022 Jan;36(1):100672. doi: 10.1016/j.trre.2021.100672.
- Monteiro OMC, Higa Júnior MG, Palhares MA, Nunes MO, Melhem MSC, Chang MR. A Rare Case of Aspergillus Mediastinitis After Coronary Artery Bypass Surgery: A Case Report and Literature Review. Am J Case Rep. 2021 Dec 15;22:e933193. doi: 10.12659/AJCR.933193.
- Valerio M, Machado M, Cedeño S, Rodríguez ML, Anaya F, Vena A, Guinea J, Escribano P, Bouza E, Muñoz P. Donor-derived invasive aspergillosis after kidney transplant. Med Mycol Case Rep. 2018 Jul 17;22:24-26. doi: 10.1016/j.mmcr.2018.07.004.
- Mueller NJ, Weisser M, Fehr T, Wüthrich RP, Müllhaupt B, Lehmann R, Imhof A, Aubert JD, Genoni M, Kunz R, Weber M, Steiger J. Donor-derived aspergillosis from use of a solid organ recipient as a multiorgan donor. Transpl Infect Dis. 2010 Feb;12(1):54-9. doi: 10.1111/j.1399-3062.2009.00463.x
