Title: Skin Lesions in a patient with Hodgkin’s Lymphoma (4 figures)
Submitted by: Anastasia Wasylyshyn, Carol Kauffman, David Brown, Stephanie Skala, Marisa H. Miceli
Institution: University of Michigan
Email: mmiceli@med.umich.edu
Date Submitted: May 6, 2020
History: A 23-year-old man with Stage IV nodular sclerosing Hodgkin’s lymphoma was admitted with fever and erythema of his left leg that had not responded to standard antibiotic therapy.
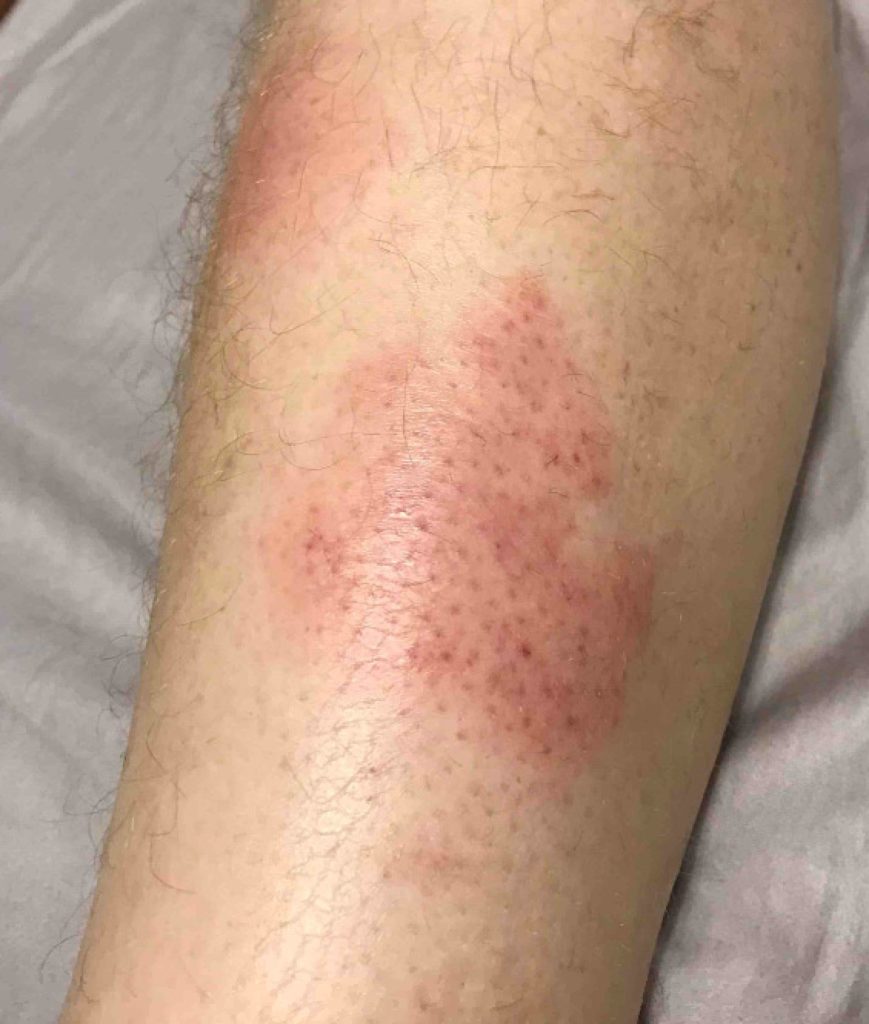
The patient has been on chemotherapy, (cycle 3 of bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) over 8 days, with the last dose three days before admission. He was admitted with fever and an expanding erythematous rash that appeared to be cellulitis on his left lower leg (Figure 1). He was initially treated with vancomycin and piperacillin-tazobactam, followed by trimethoprim-sulfamethoxazole and amoxicillin-clavulanate. He showed gradual clinical improvement, his white blood cell (WBC) count returned to normal, and he was discharged approximately 1 week later.
He was readmitted the next day with fever and worsening pain and erythema of the left lower leg. On examination, he was alert and in no distress except for a painful left lower leg. His vital signs included a BP 132/67 mmHg, pulse 97/min, Temp 37oC, respiratory rate 18/min
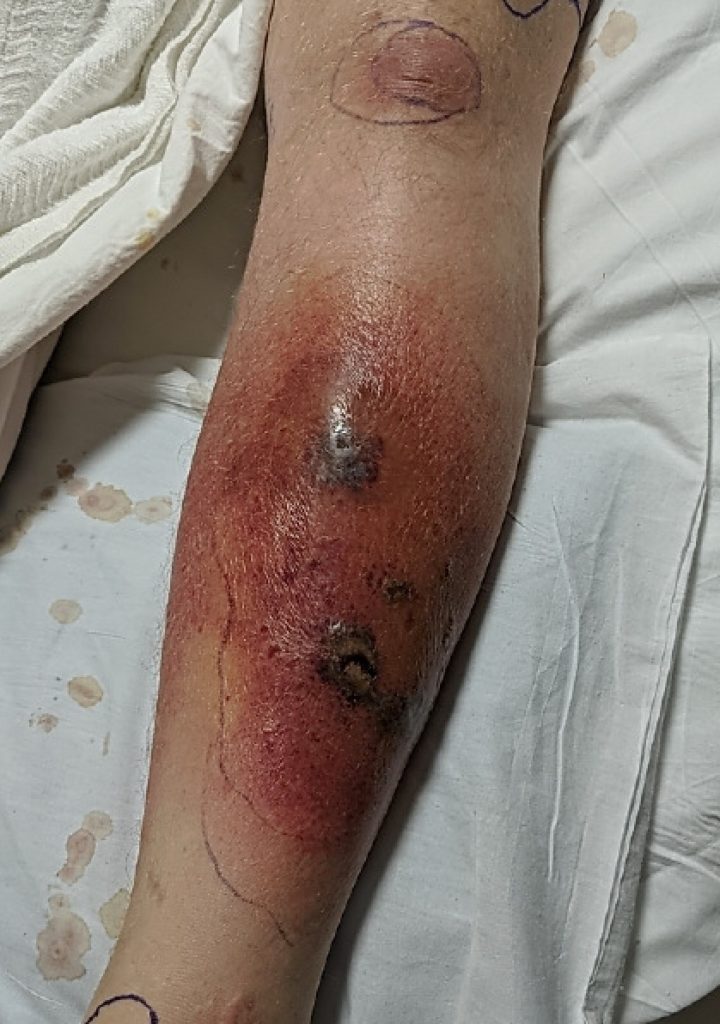
His exam was unremarkable except for his left leg which revealed erythema and swelling with several discrete areas of necrosis with hemorrhage on the left anterior lower leg. Erythema had extended beyond that noted one day earlier, and the leg was exquisitely tender to palpation (Figure 2).
Laboratory studies: WBC 44,100 cells/?L, 71% neutrophils, 1% lymphocytes, 5% monocytes, 1% eosinophils, 4% metamyelocytes, 16% myelocytes, 2% progranulocytes; hemoglobin 9.2 g/dL; platelets 51,000/?L; creatinine 1.5 mg/dL
Question 1: What are probable/possible diagnoses?
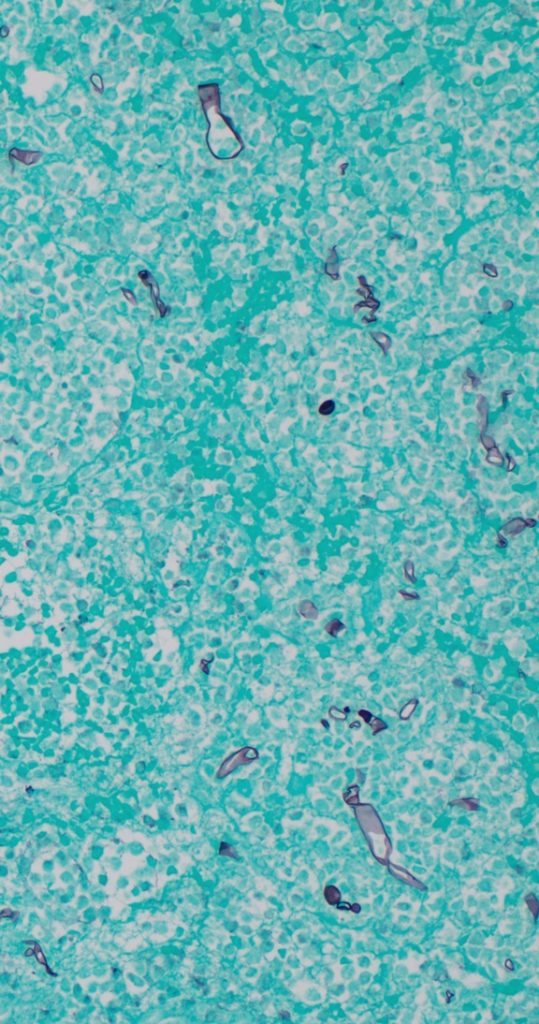
Differential diagnosis includes ecthyma gangrenosum, neutrophilic dermatosis (Sweet’s syndrome), necrotizing fasciitis, invasive mold infectionMicrobiology/Diagnostic Tests Performed: Biopsy of the lesion demonstrated tissue necrosis with numerous large non-septate hyphae on both a calcofluor-treated tissue impression and on the methenamine silver stain (Figure 3). Mucor spp., not identified to species level was isolated on blood agar within 3 days. Susceptibility studies demonstrated an amphotericin MIC <0.03 mcg/mL, isavuconazole MIC 2 mcg/mL, and posaconazole MIC 0.5 mcg/mL.
Chest computerized tomography (CT) demonstrated nodules that were consistent with lymphoma. Magnetic resonance imaging (MRI) of head and neck were unremarkable. An MRI of the left leg demonstrated cellulitis, associated fasciitis, and periosteal reaction of the lateral tibial diaphysis.
Final Diagnosis: Locally invasive mucormycosis
Question 2: What treatment is recommended in the care of this patient?
Treatment: Intravenous liposomal amphotericin B 5mg/kg daily was given for 8 weeks, along with extensive surgical debridements which were required on 2 occasions, followed by split-thickness skin grafting for closure of the surgical defect. Two months later, therapy was changed to posaconazole extended release tablets 300mg daily, which were prescribed until chemotherapy was reinstituted. In order to avoid drug-drug interactions, liposomal amphotericin B, 5 mg/kg three times a week was given for 2 months, followed by treatment to posaconazole.
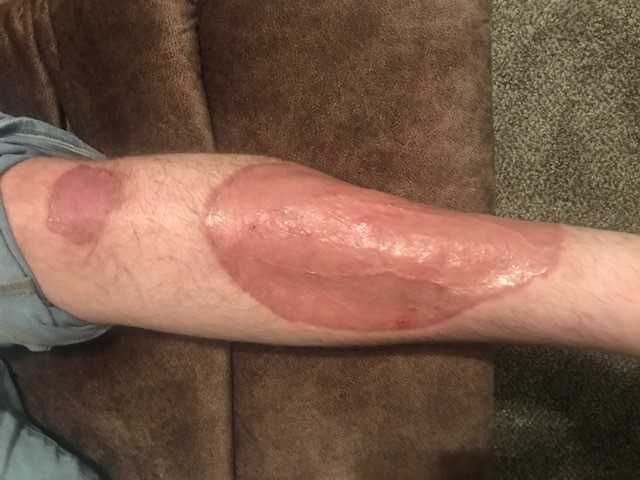
Outcome: Several months later, he feels well, has no pain and the surgical site is healed (Figure 4). A follow-up PET CT scan 3 months after chemotherapy did not show any evidence of lymphoma. It did however, show focal uptake in the left distal tibial metaphysis concerning for osteomyelitis.
Discussion:
The order Mucorales contains many different species that are ubiquitous in decaying vegetation and soil [1]. The most common genera that cause human infection are Rhizopus, Mucor, and Rhizomucor. Most of the Mucorales are opportunists, causing disease primarily in immunocompromised hosts [2]. The most common types of infections are rhino-orbital-cerebral and pulmonary. Pulmonary infection is most common in patients with hematological malignancies and in hematopoietic cell transplant recipients; rhino-orbital-cerebral involvement occurs more often in patients in whom diabetes is the major risk factor [2]. Dissemination is not uncommon in immunocompromised hosts. In addition to these typical manifestations, isolated gastrointestinal tract mucormycosis is a well-described entity, as is localized cutaneous infection, often related to trauma. In both of these entities, necrosis remains the hallmark of infection [3].
Treating mucormycosis is challenging, given the aggressive and angioinvasive nature of the organisms, as well as intrinsic antifungal resistance to most azoles and echinocandins [4]. Appropriate initial therapy is usually with a lipid formulation of amphotericin B, followed by long-term oral treatment with either isavuconazole or posaconazole [5,6]. Both drugs have the benefit of once daily dosing with a well-absorbed oral tablet formulation. Isavuconazole has the additional benefits of fewer drug-drug interactions and lower rates of QT interval shortening, as compared with QT prolongation seen with all other azoles [7]. Aggressive surgical debridement with removal of all necrotic tissue is crucial and the key to eradicating the infection [2,4]. The inability to perform extensive surgery in many patients who have pulmonary mucormycosis may contribute to the higher mortality noted in this form of mucormycosis [2].
Several aspects of this patient’s infection are novel. He is not the typical patient who is thought to be at risk for mucormycosis. Hodgkin’s disease classically is associated with defects in cell-mediated immunity and does not affect neutrophil function. There are few cases of mucormycosis reported in patients with Hodgkin’s disease [8]. His neutropenic episodes were brief (<7 days). Patients with acute leukemia receiving induction chemotherapy and hematopoetic cell transplant recipients are at higher risk for mucormycosis given the profound and prolonged neutropenia they experience. Additionally, there was no clinical or radiographic evidence of disseminated mucormycosis as an explanation for his cutaneous lesions, and hematogenous dissemination to skin is rare [2].His presentation fits best with the source of infection associated with a traumatic inoculation of the organism, but he recalled no antecedent trauma and had no findings suggesting trauma on physical examination. However, he had played baseball wearing shorts right before receiving the third cycle of chemotherapy, which finished 3 days before he developed the left lower leg lesions. Traumatic mold inoculation is most common in combat settings in soldiers who have received penetrating wounds, in persons who have survived natural disasters associated with flying debris, and in patients who have large burn wounds [3]. However, in immunosuppressed hosts, even minor trauma, such as a scratch from a pet, pressure from a wooden tongue depressor supporting an intravenous catheter or use of contaminated tape on a wound may be enough to allow inoculation of the organism. We postulate that immediately before receiving chemotherapy, it is likely that he experienced minor trauma while playing baseball and that led to inoculation of the organism.
Key References:
- Dannaoui E, Lackner M. Special Issue: Mucorales and Mucormycosis. J Fungi. 2019;6(1). pii: E6. doi: 10.3390/jof6010006.
- Roden MM, Zaoutis TE, Buchanan Wl, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41:634-54.
- Walsh TJ, Hospenthal DR, Petraitis V, Kontoyiannis DP. Necrotizing mucormycosis of wounds following combat injuries, natural disasters, burns, and other trauma. J Fungi. 2019;5(3). pii: E57.
- Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014;20 (suppl 3):5-26.
- van Burik JA, Hare RS, Solomon HF, et al. Posaconazole is effective as
- salvage therapy in zygomycosis. a retrospective summary of 91 cases. Clin Infect Dis 2006;42:e61-5.
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis 2016;16:828-37.
- Mellinghoff SC, Basetti M, Dorfel D, et al. Isavuconazole shortens the QTc interval. Mycoses. 2018;61(4):256-60.
- Nosari A1, Oreste P, Montillo M. Mucormycosis in hematologic malignancies: an emerging fungal infection. Haematologica. 2000;85(10):1068-71.
