Title: Voriconazole-induced periostitis occurring during the treatment of disseminated aspergillosis
Submitted By: Stephen Maurer, Kevin Gregg, Carol A. Kauffman, Marisa H. Miceli
Institution: University of Michigan
Email: stemaure@med.umich.edu
History:
A 59-year-old man was admitted with five days of diffuse body pain involving shoulders, arms, legs, chest, and back associated with fatigue and generalized weakness.
Notable medical history included alcoholic cirrhosis for which 3 years previously, he had received an orthotopic liver transplant that was complicated by chronic ductopenic rejection. He received a second liver transplant 5 months prior to admission. The second transplant was complicated by ascending cholangitis related to a biliary anastomotic stricture that required stenting and recurrent Klebsiella pneumoniae bacteremia.
Within several weeks of the second transplant he developed disseminated aspergillosis with pulmonary nodules, bilateral chorioretinitis, brain, renal, and subcutaneous nodules, and small abscesses near the gastroesophageal junction and the biliary and portal venous anastomoses.
He was treated with voriconazole at an initial dose of 200mg twice daily and micafungin 150 mg daily; after 14 days, the dosage of voriconazole was increased to 300mg twice daily and then to 400mg twice daily by 10 weeks because of repeatedly subtherapeutic serum trough concentrations. Genetic testing determined that he had a CYP2C19 rapid metabolizer genotype. He tolerated antifungal therapy well, and imaging demonstrated improved or stable lesions in lung, abdomen, and brain.
Physical Examination on admission:
Temperature: 36.7oC
Blood pressure: 157/89 mm Hg
Pulse: 91 beats/min.
Weight: 70.4kg (BMI 21)
General: Non-toxic appearing man in no apparent distress, not jaundiced
Pulmonary: No rales, wheezes, or rhonchi were heard on auscultation
Cardiovascular: Regular rhythm, no murmurs, rubs, or gallops
Abdomen: Soft, non-tender, non-distended, no hepatomegaly, normal bowel sounds
Musculoskeletal: No tenderness to palpation of muscles or bony structures of arms, shoulders, or legs.
Laboratory Examination on Admission:
Voriconazole serum trough concentration: 3.0 mcg/mL
White Blood Cell Count: 5,100 cells/?L
Differential: 80% neutrophils, 8% lymphocytes, 11% monocytes
Hemoglobin: 9.3 g/dL,
Serum Creatinine: 1.3 mg/dL
Aspartate aminotransferase: 45 IU/L (ref 8 – 30)
Alanine aminotransferase: 48 IU/L (ref <35)
Alkaline phosphatase: 520 IU/L (ref 40 – 116)
Serum bilirubin, total: 0.9 mg/dL (ref 0.2 – 1.2)
Notable laboratory findings at the time of diagnosis of invasive aspergillosis:
Sputum Culture: Aspergillus fumigatus
Fungal isolator blood cultures: No growth
Skin of right upper arm, punch biopsy: Septate hyaline hyphae suggesting Aspergillus species
Serum Beta-D-Glucan: 414 pg/mL (pretreatment) ? 216 pg/mL (2 weeks into treatment) ? <31 pg/mL (3 months into treatment)
Serum galactomannan (Platelia assay): <0.5 OD units
Imaging this admission:
MRI brain: Stable size of multiple small enhancing intraparenchymal lesions with single new lesion in the right precentral gyrus.
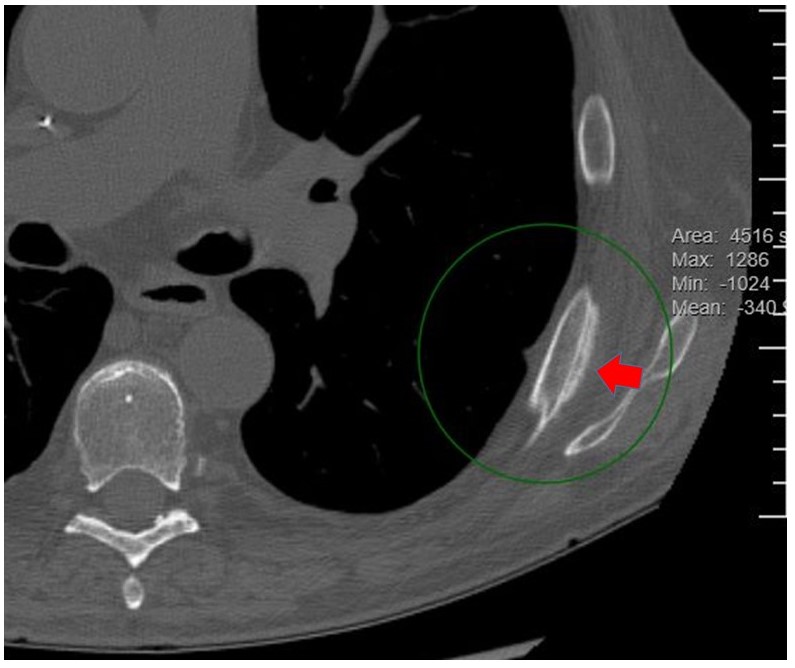
CT chest: Interval decrease in size and number of clustered and tree-in-bud nodules consistent with improving aspergillosis. New nonspecific periosteal reaction of the posterolateral left seventh and eighth ribs.
Radiographs of femur, tibia, fibula, radius, ulna, and humerus: No evident definitive diffuse periostitis.
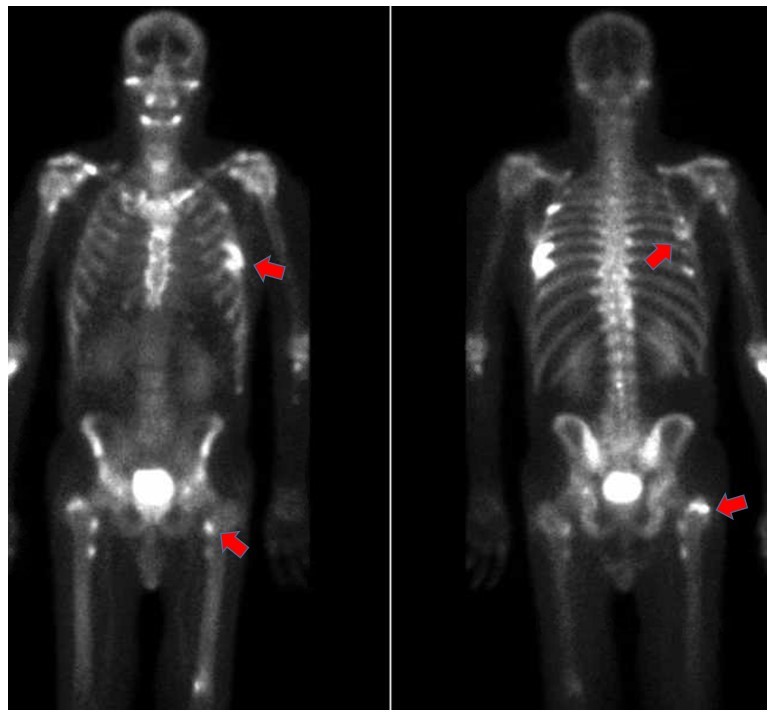
Nuclear medicine triple phase technetium-99m bone scan: Focal radiotracer uptake involving the left fourth, seventh, and eighth ribs and bilateral humeri and femurs.
Question 1: What is the most likely diagnosis?
Voriconazole-induced periostitis
Question 2: What treatment is recommended in the care of this patient:
Treatment and outcome:
The patient’s voriconazole was discontinued, and therapy switched to isavuconazole 327mg daily for treatment of disseminated aspergillosis.
Three weeks after discontinuation of voriconazole, the generalized pain and weakness had resolved. Alkaline phosphatase remained abnormal at 849 IU/L likely related to a biliary stricture. Unfortunately, serum fluoride levels were not obtained due to errors in sample collection.
Discussion:
Voriconazole is a triazole antifungal agent, the structure of which bears three fluoride groups. The relative abundance of fluoride in this agent results in an intake of 65mg of fluoride per day at a dosage of 200mg every 12 hours. Excess fluoride from drug metabolism integrates into the bone matrix as fluorapatite, which stimulates osteoblastic activity leading to periostitis [1]. Cumulative dose of voriconazole is not correlated with serum fluoride concentrations, but higher daily doses of voriconazole are positively correlated with high serum fluoride concentrations. Elevated serum fluoride concentrations in the setting of bone pain are strongly associated with periostitis on imaging [2]. The other triazole antifungals, posaconazole, isavuconazole and fluconazole, which have two fluoride moieties, have not been associated with fluorosis and periostitis; Itraconazole lacks any fluoride moieties.
Periostitis from voriconazole typically presents with diffuse bone pain that can be severe and can include palpable bony nodules in affected bone, joint swelling and tenderness, and myalgias and muscle weakness near affected bone [1]. While any bone can be affected, fluorosis most commonly involves the ulna, and the ribs and less commonly, the tibia, clavicle, femur, radius, fibula, scapula, and humerus [3].
For diagnosis, supportive laboratory features include elevated alkaline phosphatase and elevated serum fluoride concentrations. Radiographs may demonstrate multifocal periostitis with periosteal thickening in multiple, typically bilateral sites. Technetium-99m bone scintigraphy is a sensitive imaging study for diagnosis and allows imaging of the entire axial and appendicular skeleton. The findings are similar to those that are seen with metastatic malignancy and multifocal osteomyelitis [1,2].
Treatment of periostitis includes either dose reduction or discontinuation of voriconazole, which was shown to improve or resolve pain over 2 weeks to 5 months in 89% of 19 cases in one series [3]. Alkaline phosphatase and radiologic abnormalities typically normalize within 3 – 4 months [1].
There is high interpatient variability in the rate of metabolism of voriconazole due to variant alleles in the CYP2C19 enzyme, the CYP450 enzyme primarily responsible for voriconazole metabolism [4,5]. Between 2 to 30% of patients are rapid metabolizers of voriconazole caused by heterozygosity of a single nucleotide polymorphism (the*17 allele) in the gene promotor region. This results in subtherapeutic trough concentrations with standard dosing regimens and can lead to treatment failure [4,5]. With the increased daily dosing of voriconazole that is required, there is increased risk of development of periostitis, as noted in our patient.
Key References:
1. Tan I, Lomasney L, Stacy GS, Lazarus M, Mar WA. Spectrum of Voriconazole-Induced Periostitis With Review of the Differential Diagnosis. AJR Am J Roentgenol. 2019;212(1):157-165.
2. Tailor TD, Richardson ML. Case 215: voriconazole-induced periostitis. Radiology. 2015;274(3):930- 935.
3. Moon WJ, Scheller EL, Suneja A, et al. Plasma fluoride level as a predictor of voriconazole-induced periostitis in patients with skeletal pain. Clin Infect Dis. 2014;59(9):1237-1245.
4. Hamadeh IS, Klinker KP, Borgert SJ, et al. Impact of the CYP2C19 genotype on voriconazole exposure in adults with invasive fungal infections. Pharmacogenet Genomics. 2017;27(5):190- 196.
5. Moriyama B, Obeng AO, Barbarino J, et al. Clinical pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther. 2017;102(1):45- 51.
Images and Figures: