Title: A Rare Presentation of Disseminated Mucormycosis in an Immunocompetent Trauma Patient
Submitted by: Trevor Getz, MD; Haroon Alam, MS-4
Institution: Medical College of Georgia/Augusta University
Email: tgetz@augusta.edu
Date Submitted: 6/22/21
History:
HPI:
A previously healthy 63-year-old male with no significant PMH presented to the emergency department as a level 2 trauma transfer from an outside hospital after sustaining a tree trunk injury to his left lower extremity earlier in the afternoon, resulting in significant swelling. Upon transfer, patient was noted to have a possible compartment syndrome with hard thigh compartments, absent distal LLE pulses, the inability to dorsi/plantar flex his left foot and no sensation in the foot. He went to the OR where he underwent multicompartment fasciotomy and surgical repair of transected popliteal artery and vein. After surgery, the patient remained intubated and was started on CRRT. On POD#5, patient was started on pressors and amiodarone for hypotension and unstable Atrial fibrillation with a rapid ventricular rate. On POD#6, he developed leukocytosis and was empirically started on IV Vancomycin/Cefepime/metronidazole. He was taken back to the OR that same day for re-exploration and debridement. He was noted to have devitalized tissue and full thickness necrosis of the left thigh and left lower leg. Intraoperative cultures eventually grew Pseudomonas aeruginosa and Enterococcus faecalis. On POD #9, he underwent a CT Head scan due to a decline in mental status. He was also noted to have right-sided orbital proptosis and periorbital edema. Ophthalmology was consulted and a CT Orbital scan was then performed, which showed “constellation of CT imaging findings raising suspicion for elevated intraorbital pressure” but no evidence of trauma or hematoma. The following morning, the patient had developed worsening of his right eye exophthalmos and a new erythematous lesion over the right cheek which rapidly progressed over several to form a large eschar over the right malar region, despite no history of facial trauma. ENT was then consulted and performed a nasal endoscopy and obtained several punch biopsies from the rim of the facial eschar.
PMH: No chronic problems. No history of DM or immunocompromise.
Physical Examination:
General – intubated, sedated
Head/Face – large 4×5 cm eschar of the right malar region extending up towards the right eye
Eyes – periorbital edema. R worse than L with hemorrhagic chemosis, and bilateral lagophthalmos.
Nasal Cavity – extensive debris bilaterally, necrotic dusky appearance of the right and left inferior turbinates and bilateral septum, right > than left
Oral Cavity – dark lesions/ecchymosis of the mucosal upper lip
Oropharynx – intubated, palate appears dusky
Neck – soft, supple, without adenopathy
Respiratory – bilateral rhonchi,
CV – regular rate and rhythm
Extremities – swelling of the right thigh, with a dark appearing lesion over the right patellas region and necrotic lesion of the right medial foot, LLE fasciotomy incisions x4, leg malodorous with continued drainage.
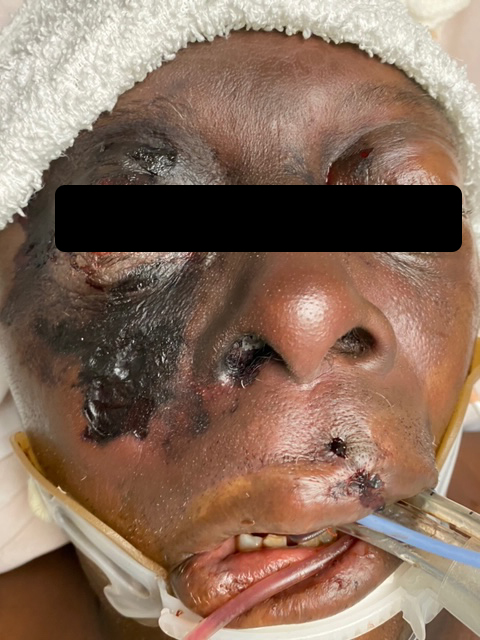
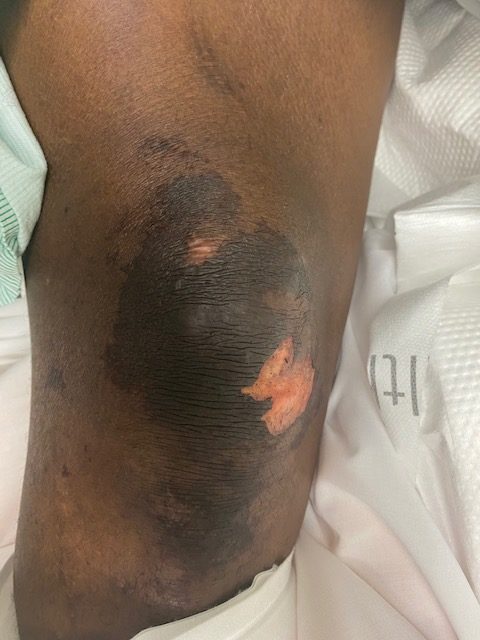
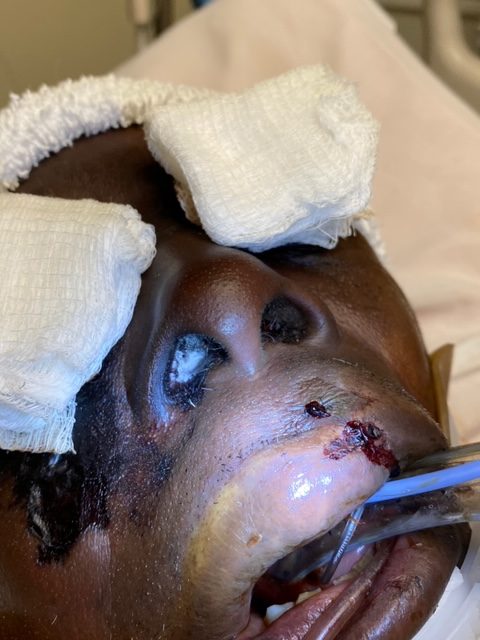
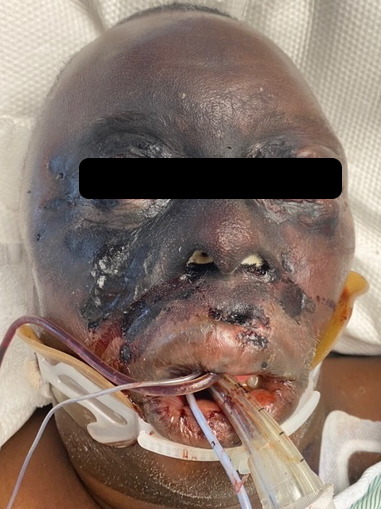
Figures 1 and 2 show the initial appearance of the facial eschars/lesions as well as extensive fungal debris within the nasal cavities. Figure 3 shows a similar eschar on the right knee. Figure 4 shows the extensive progression of necrosis over the following 24-48 hours.
Laboratory Examination: (On POD#9 at time of ENT and ID consult)
WBC: 14.1
Hgb: 10.0
Na: 135
K+: 143
Cr: 4.33
Glu: 222
Imaging:
CT Head showed “no acute intracranial abnormality or definite imaging abnormality
to account for the clinical mental status. No pathologic intracranial enhancement.”
CT Orbit showed “constellation of CT imaging findings raising suspicion for elevated intraorbital pressure. No evidence of intraorbital hematomas, including retro-ocular, orbital apex, or orbital fissural hemorrhage. No evidence of orbital masses, cavernous sinus thrombosis, or carotid-cavernous fistula. Preseptal soft tissue swelling bilaterally, greater on the right. No evidence of acute/subacute orbital fractures.”
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed:
Culture history:
07/23 Leg Tissue – Pseudomonas aeruginosa & E faecalis (susceptible to cefepime and vancomycin respectively)
07/23 BAL => Klebsiella pneumoniae (susceptible to cefepime)
07/28 Leg Swab => Pseudomonas aeruginosa
07/28 Blood Cultures => No growth
07/29 Tissue culture from bilateral inferior turbinates and right malar skin – Mucor species
Final Diagnosis: Disseminated Mucormycosis
Question 2: What treatment is recommended in the care of this patient?
Treatment: The patient was immediately started on liposomal amphotericin B and continued on IV Vancomycin, Metronidazole, and Cefepime.
Outcome: The patient had extensive fungal infection of the bilateral nasal cavities, involving bilateral inferior turbinates, nasal septum, and the palate. It also involved the right malar region and over the course of hours spread to involve the upper lip, both periorbital regions, and the left auricle. ENT had discussion with family members regarding his clinical course and treatment options. Surgical debridement would likely have been extremely morbid including likely right and possible left orbital exenteration, possible rhinectomy, lip excision, and left ear debridement. Given the aggressive and advanced nature of his disease, and his very poor prognosis, they elected not to pursue surgical debridement. Patient was placed in hospice care and died soon after.
Discussion: Mucormycosis is a life-threatening disease caused by filamentous molds of the order Mucorales. Infection can occur from ingestion of contaminated food, inhalation of spores into the nasal cavity or lungs, or via inoculation into wounds. Mucor often manifests with a high propensity for angioinvasion, which can lead to thrombosis and necrosis of tissue. In developed countries, mucormycosis most commonly presents in immunocompromised patients, i.e. transplant patients, diabetic patients, especially those in ketoacidosis, patients with malignancies, patients receiving chemotherapy, and burn patients. Mucormycosis in patients without underlying disease or immunocompromise is rare but has been reported in 6-10% of cases. The morbidity rate for IFS has been found to be up to 80% so immediate treatment is necessary. The diagnosis of mucormycosis relies upon histopathology and culture. Blood tests are of limited diagnostic value and blood cultures can often be negative even in cases of disseminated disease. Mucor has a distinct histological appearance, with irregular, non-septate hyphae that branch at right angles.
Mucormycosis can be classified based on its anatomic involvement into the following categories: Rhino-orbital-cerebral mucormycosis (ROCM), Cutaneous, Pulmonary, Gastrointestinal, Disseminated, and Uncommon Sites.
Due to its fulminant course and high morbidity, treatment of mucormycosis should begin immediately if suspected. Management typically involves wide surgical resection of necrotic and infected tissue along with liposomal amphotericin B. The newer triazoles, posaconazole and isavuconazole may also be effective in patients intolerant of liposomal amphotericin B. Just as important, is the control of the underlying immunosuppressive disease, correction of acidosis and blood glucose in diabetic ketoacidosis or decreasing dosage of immunosuppressive agents in neutropenic or solid organ transplant recipients.
This case is unusual in that the patient developed disseminated mucormycosis without any history of immunosuppression. The patient appeared to have been in relatively stable health prior to his inciting trauma and hospital course. The patient had no history of malignancies, transplants, or extensive use of chemotherapeutic drugs or steroids. While the patient’s glucose levels were at times elevated during his hospital course (low 200s), the majority of the time his CBGs remained <200. This is well under the usual range of hyperglycemia seen in patients with mucormycosis secondary to DKA or hyperosmolar hyperglycemic syndrome. He therefore likely developed cutaneous mucormycosis either from his inciting leg trauma, which disseminated to involve the nasal cavities and face or possibly had unrecognized facial trauma which served as the initial inoculation site that then spread to involve surrounding facial structures and cutaneous surfaces of the face and lower extremities.
Cocanour et al. reviewed cases of cutaneous mucormycosis in trauma patients. A 9-year retrospective review identified eleven patients with biopsy- or culture-proven mucormycosis. Nine patients were victims of blunt trauma and two patients had burns measuring greater than 50% TBSA. None of the patients had underlying disease or immunosuppression prior to their injuries. Four of the patients developed phycomycotic gangrenous cellulitis of the head, the trunk, or both and all four ultimately died of mucormycosis. They concluded that “because of underlying disease, contaminating wounds, antibiotic use, or immunocompromise secondary to shock and sepsis, trauma patients are at risk of developing mucormycosis.”
Giacobbe et al. also reviewed case reports and case series of mold infections of traumatic wounds. They noted that while “most of the published clinical experience and research regarding wound mold infections following traumatic injuries regards soldiers and natural disasters, these infections may also be observed in other immunocompetent individuals with trauma and soil contamination (e.g., agricultural or automotive injuries). In such cases, diagnosis and treatment could be delayed due to lack of awareness of mould infections during routine care. Clinical signs such as necrosis and reduced response to antibacterial therapy should be promptly identified to rapidly start an adequate laboratory workflow and empirical antifungal therapy in rapidly evolving cases.”
Overall, this case serves as an important reminder to physicians to always have a high index of suspicion in patients with any signs or symptoms of mucormycosis due to its fulminant and potentially fatal course. While rare, mucormycosis can occur in immunocompetent individuals, especially trauma patients.
Key References:
- Damante JH, Fleury RN, Oral and rhinoorbital mucormycosis: Case report, Journal of Oral and Maxillofacial Surgery, Volume 56, Issue 2, 1998, Pages 267-271, ISSN 0278-2391, https://doi.org/10.1016/S0278-2391(98)90883-7.
- Reid G, Lynch JP 3rd, Fishbein MC, Clark NM. Mucormycosis. Semin Respir Crit Care Med. 2020 Feb;41(1):99-114. doi: 10.1055/s-0039-3401992. Epub 2020 Jan 30. PMID: 32000287.
- Petrikkos G, Skiada A, Lortholary O, Roilides E, Walsh TJ, Kontoyiannis DP. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54 Suppl 1:S23-S34. doi:10.1093/cid/cir866
- Skiada A, Petrikkos G. Cutaneous mucormycosis. Skinmed. 2013;11(3):155-160.
- Cocanour CS, Miller-Crotchett P, Reed RL 2nd, Johnson PC, Fischer RP. Mucormycosis in trauma patients. J Trauma. 1992;32(1):12-15. doi:10.1097/00005373-199201000-00003
- Giacobbe DR, Riccardi N, Vena A, Bassetti M. Mould Infections of Traumatic Wounds: A Brief Narrative Review. Infect Dis Ther. 2020;9(1):1-15. doi:10.1007/s40121-020-00284-8
