Title: Survival of cerebral scedosporiosis after allogeneic stem cell transplantation
Submitted by: Jannik Stemler, MD; Philipp Koehler, MD, FECMM; Oliver A. Cornely, MD, FECMM, FIDSA
Institution: University of Cologne, Faculty of Medicine and University Hospital Cologne, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf (CIO ABCD), Excellence Center for Medical Mycology (ECMM), Cologne, Germany and University of Cologne, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), Cologne, Germany.
Email: jannik.stemler@uk-koeln.de
Date Submitted: 01/04/2020
History:
A 65-year-old man with FLT3-mutated and complex aberrant karyotype acute myelogenous leukemia presented himself in the outpatient clinic for follow-up after allogeneic stem cell transplantation. The patients’wife reported a slight character change and memory loss over a period of one week.
In his clinical course, the patient had persisting neutropenic fever under broad-spectrum antibiotic therapy during allogeneic stem cell transplantation. Chest CT revealed probable invasive pulmonary aspergillosis (IPA) which occurred during posaconazole prophylaxis. He received treatment with liposomal amphotericin B (LAmB). Defervescence occurred upon recovery from aplasia and engraftment of cells was as expected. Follow up chest CT showed decreasing pulmonary lesions. Therefore posaconazole tablets were administered and LAmB stopped. He was then discharged thirty-five days after transplant.
Past Medical History:
- Diabetes mellitus
- Arterial hypertension
- Angioedema due to ACE-inhibitor
- Pacemaker implantation (DDD) – AV-block second degree (type Mobitz)
- Chronic hepatitis B
Physical Examination:
The patient had decreased cognitive function including retardation of motoric function and speech. He was disorientated to time and space. Furthermore, beginning left-sided hemiparesis was observed. No further neurological symptoms were evaluated at that point. The patient was not febrile. Vital signs were normal.
Laboratory Examination (range):
– WBC: 4.83 x1E9/l (ref. 4.4 – 11.3)
– RBC: 3.3 x1E12/l (ref. 4.5 – 5.9)
– Hb: 9.6 g/dl (ref. 13.5 – 18.0)
– Hct: 28 % (ref. 42 – 50)
– PLT: 47 x1E9/l (ref. 150 – 400)
– CRP: 8.5 mg/l (ref. <5,0)- GM:0.3 ODI (<0.5)
Microbiology/Diagnostic Tests Performed:
- Blood cultures: negative for bacteria and fungi
- Galactomannan in serum: <0.5 ODI; repeatedly
- Cranial CT: lesion in the right frontal lobe
- Cerebrospinal fluid: visually clear, 7 cells, no lactate, protein 0.87g/l (Ref 0.15-0.45 g/l); cultures of CSF remained negative; fungal and viral PCR were not performed
Cranial magnetic resonance imaging (MRI):
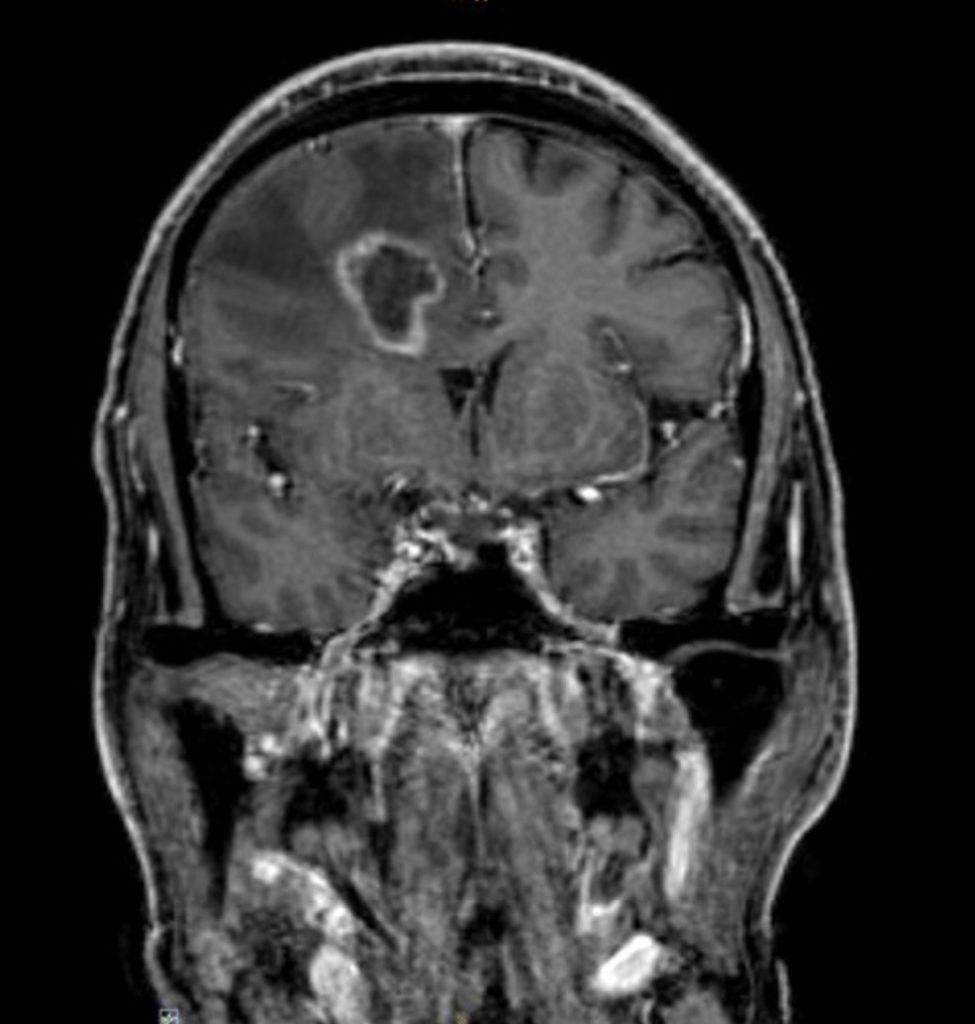
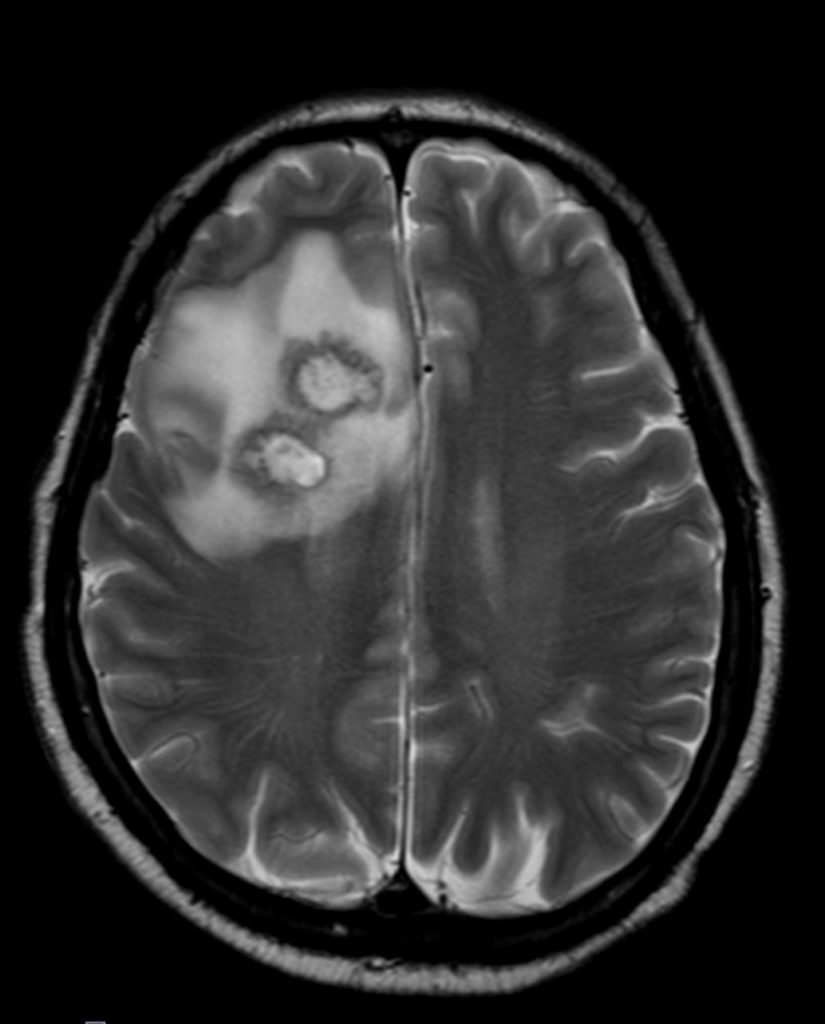
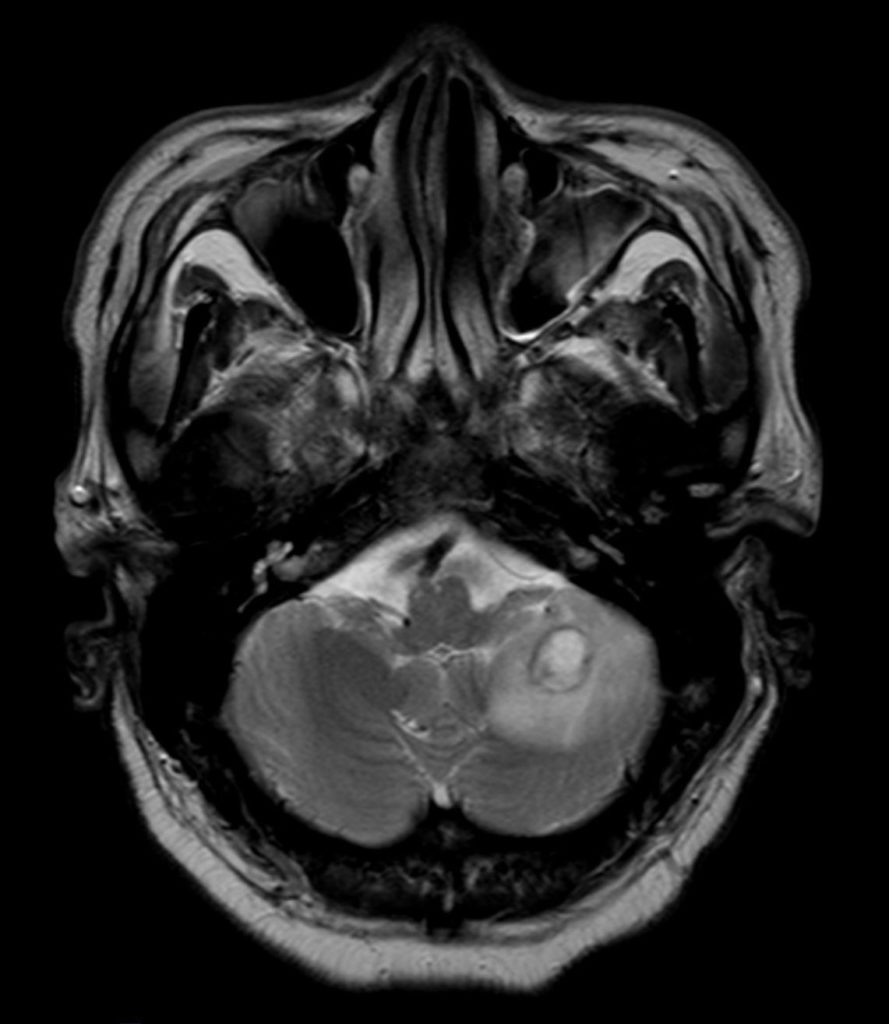
Stereotactic biopsy:
Results of culture and rDNA D1/D2 and ITS1/ITS2-PCR: Scedosporium species (Scedosporium apiospermium)
| Results from Antifungal susceptibility testing | ||
| Drug | Minimal inhibitory concentration (MIC) | Interpretation |
| Itraconazole | 4 mg/l | No breakpoints established |
| Amphotericin B | >32 mg/l | No breakpoints established |
| Voriconazole | 0.064 mg/l | No breakpoints established |
| Posaconazole | 4 mg/l | No breakpoints established |
Clinical course.The patient was admitted to our intermediate care ward. Blood cultures remained negative. Cranial CT showed a lesion suspicious of an abscess in the right frontal lobe. Empiric broad-spectrum antibiotic therapy was initiated (Piperacillin/Tazobactam and Metronidazole). MRI revealed multiple abscesses in the right frontal, parietal lobe and cerebellum with midline shift to the left hemisphere. Subsequently, stereotactic biopsy was performed. Culture revealed filamentous fungi which was later identified as Scedosporium apiospermium confirmed by PCR analysis. Antifungal treatment was initiated. Due to disseminated infection, it was decided not to perform surgical treatment or drainage of cerebral abscesses.
Final Diagnosis: Cerebral Scedosporiosis
Question 2: What would be the recommended treatment?
Treatment:
The current ESCMID guidelines recommend first line treatment with voriconazole providing an A recommendation for immunocompromised patients. Surgery is advised if feasible and lesion(s) remain localized (1).
We initiated voriconazole 6 mg/kg b.i.d intravenously. Therapeutic drug monitoring showed voriconazole serum levels of 2,1 mg/l. Subsequently, voriconazole was reduced to 5 mg/kg b.i.d. i.v. on day 3 of treatment. Results of antifungal susceptibility testing showed low MIC for voriconazole and treatment was continued. Upon discharge, voriconazole was changed to oral administration and patient was followed weekly. Serum levels remained rather stable and no further dose adaptions were required. Treatment was continued for six months and cranial imaging showed improvement of cerebral lesions.
Outcome:
During inpatient stay, the patient improved slowly with his neurological dysfunction. He was discharged on day 45. The patient recovered without sequelae of the cerebral infection. During the six months of follow-up, his clinical course was unremarkable. MRI were not performed anymore due to cardiac device, but repeated CT scans did not show any further lesions. Unfortunately, the patient died eight months after diagnosis due to sepsis because of S. aureus bacteremia.
Discussion: (500 words)
Scedosporiosis/Lomentosporiosis is an emerging, rare fungal infection mostly affecting immunosuppressed patients with underlying hematological malignancy or solid organ transplant. Immune-healthy hosts with a history of trauma or near-drowning events can also be affected. Clinical manifestation varies but disseminated infection and CNS involvement are reported frequently and determine prognosis. Mortality of scedosporiosis is extremely high and rises up to 75% in allogeneic HSCT patients (2). However, antifungal prophylaxis in at-risk patients is particularly difficult due to pan-resistance to many antifungals.
In our case, S. apiospermium was identified via culture-based methods and rDNA ITS sequencing which represents the gold-standard for clinical diagnosis and species distinction. Accuracy of matrix-laser desorption/ionization mass spectrometry (MALDI-TOF/MS) is comparable. Differentiation of Lomentosporaspp. is important and can affect treatment decision. Direct microscopy and histological analysis can be implemented, however, it is difficult to distinguish Scedosporium/Lomentospora from Aspergillus or Fusariumin tissue since all present hyaline, septated hyphae with dichotomous branching (3, 4).Once diagnosis was established in our patient, voriconazole was started as recommended by guidelines. Treatment duration should be up to six months, depending on site of infection and therapeutic drug monitoring is advised (1). Voriconazole is able to pass the blood-brain barrier and shows best in vitro activity against Scedosporium spp. Patients treated with voriconazole had longer mean survival and lowest mortality. Synergistic effects of co-administration with other antifungals such as terbinafine or amphotericin B have been observed in vivo (2, 5). Antifungal susceptibility testing is essential due to variable activity against these fungi. Novel antifungal agents such as Olorofim or Fosmannogepix are promising as future treatment options for scedosporiosis since they show good in vitro activity and clinical efficacy studies are ongoing (5, 6).
Despite antifungal prophylaxis with posaconazole, our patient developed two breakthrough IFI, the first one as probable IFI due to Aspergillus spp., later a proven IFI due to Scedosporium apiospermium. Antifungal prophylaxis becomes more complex in modern AML therapy. On the one hand this is due to emerging fungal infections with resistance to antifungal agents used for prophylaxis and on the other hand, novel drugs comprising increased interaction potential with triazoles are more frequently administered. Both apply in the reported patient who had FLT3-mutated AML and was treated with midostaurin 50mg b.i.d. during induction remission chemotherapy. Posaconazole is an established standard of care as antifungal prophylaxis. However, a 50% dose-reduction of midostaurin while concomitant administration of posaconazole and therapeutic drug monitoring of both drugs is a future option in sense of personalized-medicine.
While scedosporiosis remains a severe opportunistic infection with an extremely poor prognosis in the immunocompromised host, our patient survived cerebral scedosporiosis despite immunosuppression and CNS involvement.
Key References:
1. Tortorano AM, Richardson M, Roilides E, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. 2014 Apr;20 Suppl 3:27-46.
2. Seidel D, Meissner A, Lackner M, et al. Prognostic factors in 264 adults with invasive Scedosporium spp. and Lomentospora prolificans infection reported in the literature and FungiScope((R)). Crit Rev Microbiol. 2019 Feb;45(1):1-21.
3. Ramirez-Garcia A, Pellon A, Rementeria A, et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018 Apr 1;56(suppl_1):102-25.
4. Lackner M, Klaassen CH, Meis JF, van den Ende AH, de Hoog GS. Molecular identification tools for sibling species of Scedosporium and Pseudallescheria. Med Mycol. 2012 Jul;50(5):497-508.
5. Lamoth F, Kontoyiannis DP. Therapeutic Challenges of Non-Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob Agents Chemother. 2019 Nov;63(11).
6. Wiederhold NP, Law D, Birch M. Dihydroorotate dehydrogenase inhibitor F901318 has potent in vitro activity against Scedosporium species and Lomentospora prolificans. Journal of Antimicrobial Chemotherapy. 2017;72(7):1977-80.
