Title: Candida auris Bloodstream Infection complicated by Micafungin-Induced Allergic Rash
Submitted by: David Brower1, Maura Connorton1, Divisha Sharma MD2, Jose Vazquez MD 2
Institution: Medical College of Georgia.
WellStar MCG at Augusta university, Dept. of Medicine, Division of Infectious Disease
Email: dbrower@augusta.edu
Date Submitted: 9/12/24
History:
A 34-year-old male, with a history of schizophrenia and multiple self-inflicted abdominal injuries requiring exploratory laparotomies, was transferred from a state medical prison to the emergency department. He presented with worsening abdominal pain, generalized weakness, and several hours of non-bloody, non-bilious vomiting. The patient was 14 days post-operative from gastro-cutaneous fistula revision surgery and was receiving total parenteral nutrition (TPN) at the time of presentation.
Upon examination, the patient was febrile at 38°C, tachycardic, and diaphoretic, though he appeared to be in no acute distress. His mucous membranes were dry, and a peripherally inserted central catheter (PICC) was noted in the right upper extremity, which showed no signs of ecchymosis, erythema, or tenderness. The abdominal incision was clean, dry, and intact. Cardiopulmonary examination was unremarkable, and there was no evidence of peripheral edema.
Laboratory results revealed leukocytosis of 13,000/mm³, elevated lactic acid at 2.5 mmol/L, mild electrolyte imbalances, and slightly elevated alanine transaminase (ALT) at 58 U/L, with alkaline phosphatase (ALP) at 438 U/L. Initial sepsis workup included blood cultures from two sites, and the patient was empirically treated with intravenous fluids and vancomycin. A CT scan of the abdomen and pelvis demonstrated stable post-operative changes without evidence of visceral abscesses or fluid collections. The patient was discharged back to the state medical prison with instructions to continue current management, including fluid resuscitation and antibiotics, and to return if symptoms worsened.
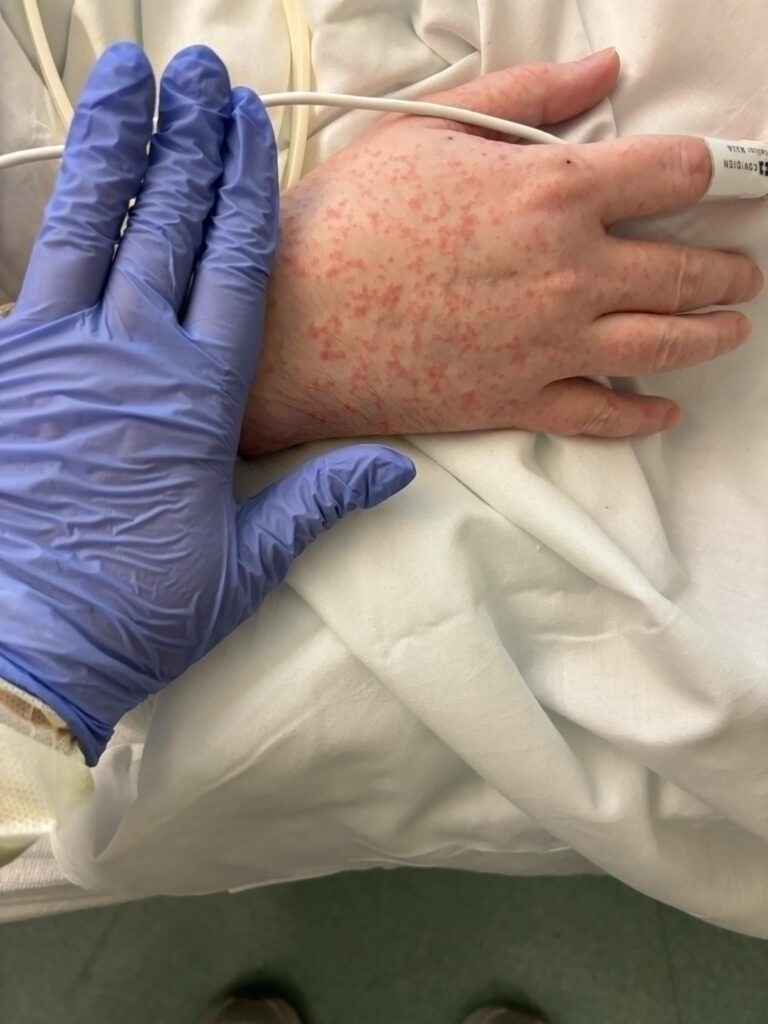
Two days later, the patient returned to the emergency department with persistent symptoms and a malfunctioning PICC line. The previously drawn blood cultures returned positive for Candida auris, confirming untreated invasive candidiasis. The patient was admitted to the hospital, and intravenous micafungin therapy was initiated, while the PICC line was removed. Despite initial clinical improvement, the patient developed a non-blanching maculopapular rash on his arms and legs bilaterally within two days of starting micafungin (Figure 1). He was afebrile, and repeat blood work showed resolution of leukocytosis, mild peripheral eosinophilia, and normal kidney and liver functions.
The patient recalled receiving a three-week course of micafungin for Candida albicans bloodstream infection two months earlier, during which he experienced a similar rash. At this time, antifungal susceptibility results were pending. Given the potential for C. auris resistance to other antifungal classes, the patient was switched to another echinocandin, rezafungin. Following discontinuation of micafungin, the rash subsided. Repeat surveillance blood cultures remained negative, and susceptibility testing later confirmed susceptibility micafungin and rezafungin. The patient received two doses of rezafungin, one week apart, and tolerated the treatment well without any adverse reactions. He was subsequently discharged in stable condition.
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed:
Blood Culture- multiplex PCR and BCID: Candida auris
Final Diagnosis: C. auris Bloodstream infection, Micafungin allergy
Question 2: What treatment is recommended in the care of this patient?
Treatment: Echinocandins, are the recommended first line treatment for C. auris. However, considering the patient’s cutaneous reaction to micafungin, his treatment was changed to rezafungin with the hope of avoiding the cross-reaction.
Outcome: The patient recovered from his C. auris bloodstream bacteremia and his rash subsided.
Discussion:
This report emphasizes the challenges in the antifungal management of Candida auris infections.
Candida auris, was initially isolated in Japan in 2009 and initially described in the United States in 2013.1 Its incidence has been rising rapidly in recent years. It increased by 95% in 2021, and in 2022 the CDC reported over 2000 clinical cases and almost 6000 positive screenings. The total number of cases detected increased sharply in early 2023, suggesting that the fungus is becoming even more prevalent.2 Unlike other Candida species, C. auris is not common constituent of the gastrointestinal microbiome.3 It has a predilection for the groin and axillary regions, and is spread from person to person, either through direct skin to skin contact or through surfaces. 4 It typically causes outbreaks in healthcare facilities and prisons, leading to serious disease in patients least equipped to fight off the infection.
C. auris’ extensive resistance profile has made it challenging to treat. Most isolates are not susceptible to azoles, and some samples have been resistant to amphotericin B and echinocandins as well.5 Due to this and its predilection for already sick patients, C. auris has had reported crude mortality of up to 35%, although studies have been limited due to the small number of cases.6 Because of its typical antimicrobial susceptibilities and serious mortality risk, the CDC recommends treating C. auris infections with echinocandins empirically until susceptibilities can be established. 7
Our case required considerably nuanced clinical decision making. As the guidelines recommended, we started our patient on an empiric echinocandin, micafungin. However, within a few days, the patient developed diffuse severe non-blanching maculopapular rash on his bilateral upper and lower extremities. Since the patient reported a similar rash previously when he was given micafungin several months previously, and such a rash is seen as a common side effect of echinocandins, including micafungin, we believed he had previously been sensitized to micafungin and thus caused his rash.
Several therapeutic options were considered in the management of this patient. First, we considered switching to liposomal amphotericin B since resistance rates for the drug are acceptable, ranging from 8% to 43%of isolates, and it is recommended as the second line treatment.8-10 However, since amphotericin B has its own significant side effect profile11 and can be resistant in C. auris, we decided against initiating the polyene treatment. Another option was “treating through” the rash. Although there is no evidence regarding the continued management with micafungin and its possible complications there is evidence that, when necessary, it may appropriate to continue to treat with the offending antimicrobial.12 Even though it would likely have been safe to continue micafungin, our patient was distressed. The patient refused to continue therapy and thus we decided to change his antifungal to rezafungin.
Rezafungin offered many unique advantages in this case. First, our patient’s susceptibilities had not yet returned, so an echinocandin was likely susceptible. It has strong in vitro and in vivo13 efficay against C. auris and has a distinguished safety profile with minimal side effects. 14 Additionally, its high front-loaded dosing creates high plasma concentrations leading to a half-life of over 150 hrs after the second dose, along with excellent tissue penetration and anti-biofilm properties mean that the drug can be dosed once weekly.14 Furthermore, rezafungin has minimal metabolism in the body. For this patient who resides in a medical prison, this meant that he could have his PICC line removed before discharged.
C. auris is an increasingly common cause of bloodstream infections worldwide. Managing this infection will be a considerable challenge for infectious disease physicians in the coming years. Certainly, there will be many complications and adverse reactions, and we hope this case will provide guidance and confidence in overcoming them.
Key References:
1. Vallabhaneni S. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus—United States, May 2013–August 2016. MMWR Morb Mortal Wkly Rep. 2016;65.
2. Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. Journal of Antimicrobial Chemotherapy. 2018;73(4):891-899.
3. Day AM, McNiff MM, da Silva Dantas A, Gow NAR, Quinn J. Hog1 regulates stress tolerance and virulence in the emerging fungal pathogen Candida auris. mSphere. 2018;3(5):10-1128.
4. Biswal M, Rudramurthy SM, Jain N, et al. Controlling a possible outbreak of Candida auris infection: lessons learnt from multiple interventions. Journal of Hospital Infection. 2017;97(4):363-370.
5. Tsay S, Kallen A, Jackson BR, Chiller TM, Vallabhaneni S. Approach to the Investigation and Management of Patients with Candida auris, an Emerging Multidrug-Resistant Yeast. Clinical Infectious Diseases. 2018;66(2):306-311. doi:10.1093/cid/cix744
6. Morales-López SE, Parra-Giraldo CM, Ceballos-Garzón A, et al. Invasive infections with multidrug-resistant yeast Candida auris, Colombia. Emerg Infect Dis. 2017;23(1):162.
7. Centers for Disease Control. Clinical Care of C. auris infections. https://www.cdc.gov/candida-auris/hcp/clinical-care/index.html.
8. Chowdhary A, Prakash A, Sharma C, et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: role of the ERG11 and FKS1 genes in azole and echinocandin resistance. Journal of Antimicrobial Chemotherapy. 2018;73(4):891-899.
9. Tsay S. Notes from the field: ongoing transmission of Candida auris in health care facilities—United States, June 2016–May 2017. MMWR Morb Mortal Wkly Rep. 2017;66.
10. Lone SA, Ahmad A. Candida auris—the growing menace to global health. Mycoses. 2019;62(8):620-637.
11. JP UTZ. Amphotericin B toxicity; general side effects. Ann Intern Med. 1964; 61:340-343.
12. Trautmann A, Benoit S, Goebeler M, Stoevesandt J. “Treating through” decision and follow-up in antibiotic therapy-associated exanthemas. J Allergy Clin Immunol Pract. 2017;5(6):1650-1656.
13. Hager CL, Larkin EL, Long LA, Ghannoum MA. Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model. Journal of Antimicrobial Chemotherapy. 2018;73(8):2085-2088. doi:10.1093/jac/dky153
14. Sharma D, Vazquez JA. An evaluation of Rezafungin: the latest treatment option for adults with candidemia and invasive candidiasis. Expert Opin Pharmacother. 2024;25(4):339-347. doi:10.1080/14656566.2024.2331775
