Title: Invasive Intracranial Aspergillosis in an Immunocompetent Patient: Diagnostic Dilemmas and Multidisciplinary Management
Submitted by: August Anderson MS, Jesse Raposa DO, Divisha Sharma MD, Jose Vazquez MD, Hasan Samra MD, Ashley Huggett DO, Andrew Chao MD
Institution: WellStar Medical College of Augusta Hospital at Augusta University
Email: auganderson@augusta.edu
Date Submitted: 6/10/24
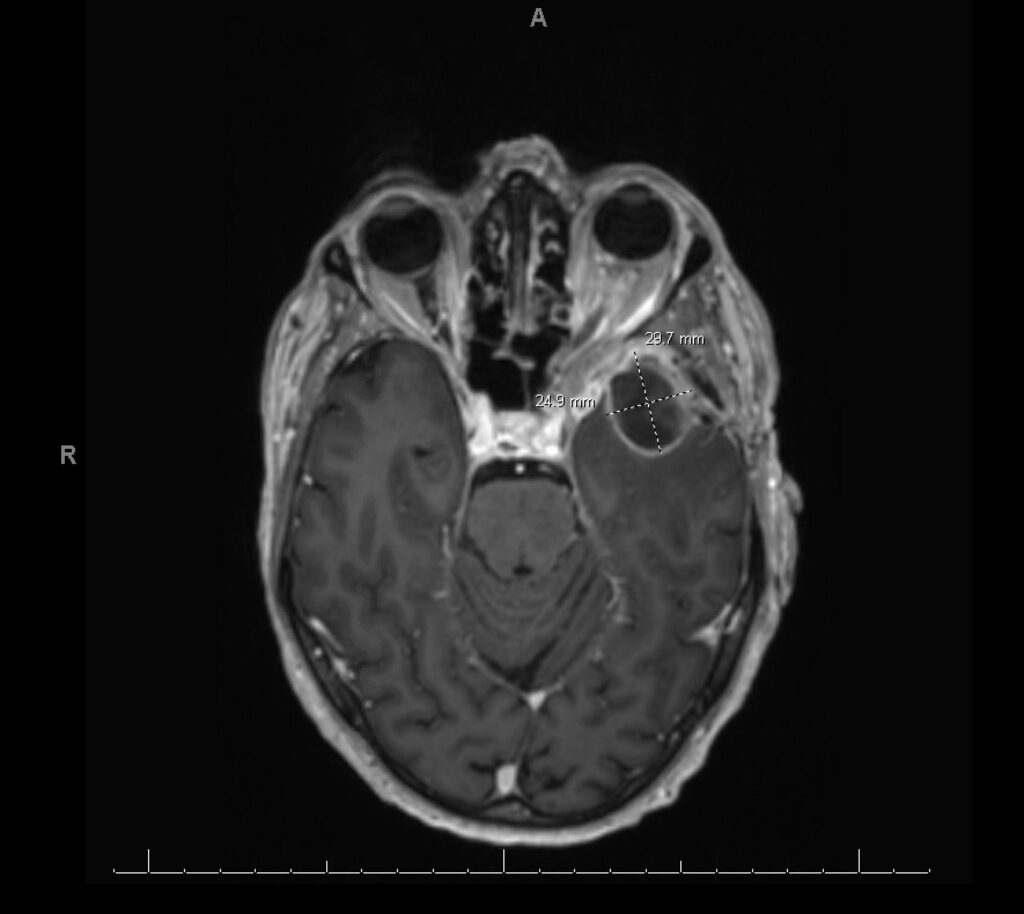
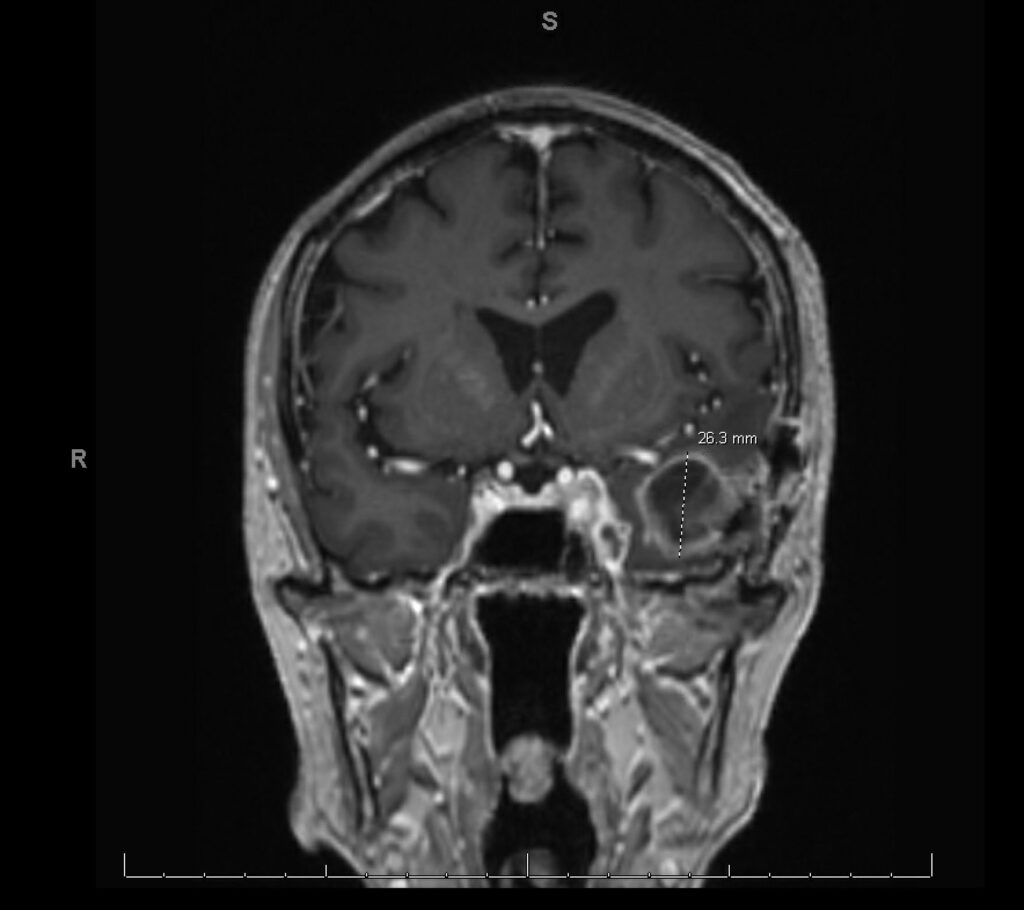
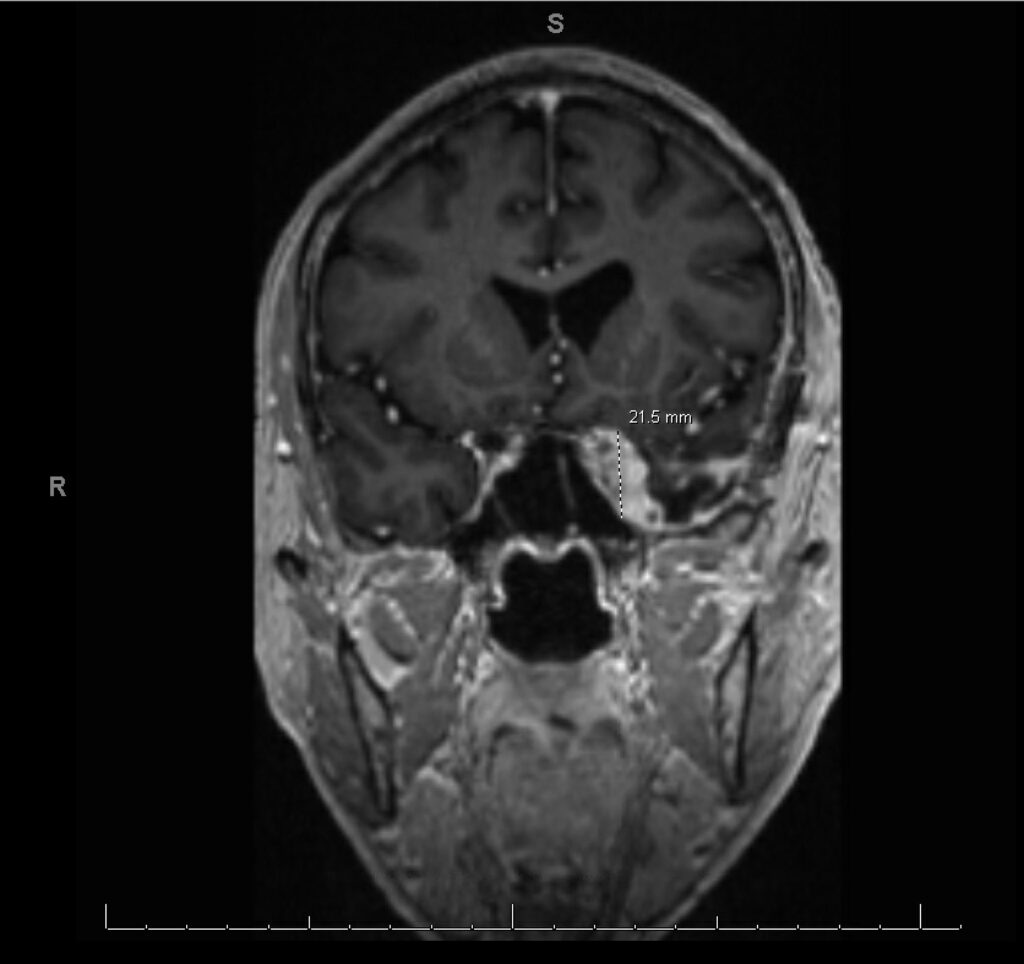
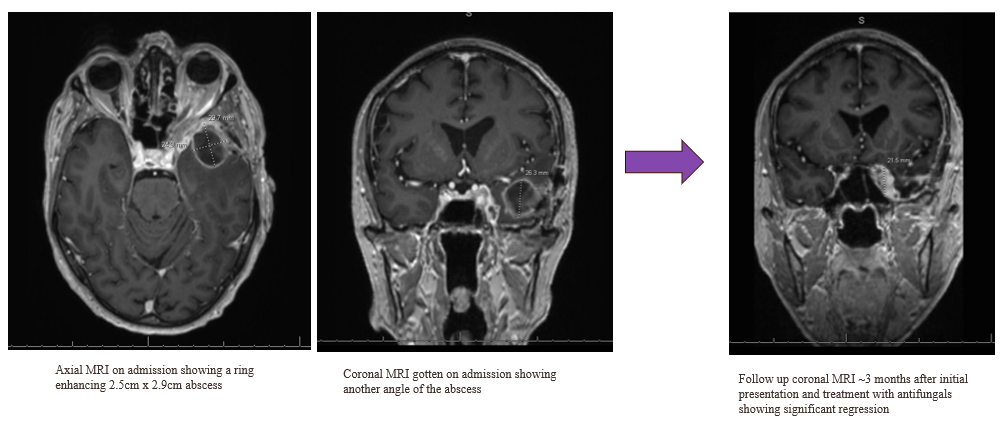
History: We present a 69-year-old male with a history of hypertension and a prior history of COVID-19 infection one year ago, who presented to the ER with persistent symptoms of headache, sinus pressure, and progressive left-sided visual loss for one year. Imaging studies including CT scans revealed sinusitis with erosive bony changes. Subsequent MRI of the brain demonstrated a large enhancing lesion adjacent to the anterior clinoid process, accompanied by multiple cystic lesions within the left frontal and temporal lobes, as well as orbital bony edema (see Image II and III).
Physical Examination: On his initial physical exam, neurological exam was normal and cranial nerves were intact except a dense field cut to confrontational testing in the upper left optic nerve territory nerve territory. Mild erythema and swelling over the left zygomatic arch were noted.
Laboratory Examination: The patient presented with mild leukocytosis (12,500/mm³) and a hemoglobin level of 12.4 g/dL. Platelet count and white cell differential were within normal limits. Serum chemistry showed a glucose level of 138 mg/dL with normal electrolytes and an HbA1c of 5.1%.
Question 1: What are probable/possible diagnoses?
The patient reported significant weight loss, headaches, and vision loss. MRI findings revealed a heterogeneously enhancing multi-cystic mass involving the paranasal sinus, sphenoid, cavernous sinus, frontal, and temporal lobes, with significant involvement of the temporal lobe. The differential diagnosis for these findings includes:
1. Malignant Carcinoma: This could be indicative of a primary or metastatic malignancy infiltrating these areas.
2. Non-infectious Inflammatory Processes: Conditions such as sarcoidosis or granulomatosis with polyangiitis (Wegener’s) could present similarly.
3. Infectious Etiologies: As imaging also showed significant thickening and enhancement of the left mastoid sinus with bilateral air-fluid levels and radiographic evidence of communication between the sinuses and intracranial space. This supports infectious etiology like bacterial sinusitis with intracranial extension, which may lead to abscess formation or osteomyelitis. Another important differential includes fungal sinusitis, particularly with Nocardia, Aspergillus or Mucor species, a consideration, especially in immunocompromised patients.
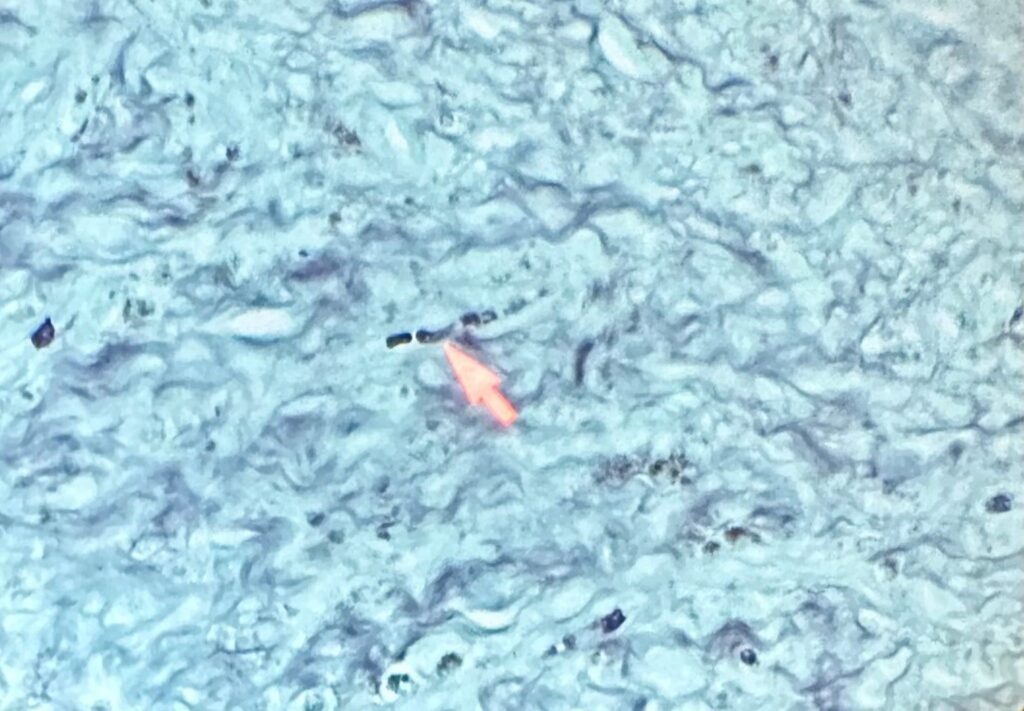
Microbiology/Diagnostic Tests Performed: During Functional Endoscopic Sinus surgery (FESS), bacterial culture of purulent material from the sinus identified Pseudomonas aeruginosa and Cutibacterium avidum. Hence diagnosis of bacterial sinusitis was established initially. Histopathology specimen from the anterior and left temporal lobe biopsy showed necrotizing granulomatous inflammation with fungal organisms – mycetoma. GMS (Gomori Methenamine-Silver) stain visualized fungal elements with septate hyphae with acute-angle branching. A bacterial and fungal culture was requested during revision craniotomy which revealed growth of Aspergillus fumigatus species complex.
Final Diagnosis: Invasive Fungal Sinusitis with Intracranial Abscess, Aspergillosis in an immunocompetent patient.
Question 2: What treatment is recommended in the care of this patient?
Treatment: Serial surgical debridement, liposomal amphotericin B IV daily (5 mg/kg) initially x 7 days, and then transition to isavuconazonium sulfate 372 mg PO daily for 1 year.
Outcome: The patient responded well and recovered to baseline except for permanent left side vision loss, left facial numbness, taste and smell. He follows up monthly with infectious diseases, neurosurgery, ENT, and monthly MRIs (Magnetic Resonance Imaging) to evaluate any disease progression.
Discussion: Despite the patient’s lack of major predisposing factors for invasive fungal infections, such as an apparent absence of known immunocompromising conditions, the severity and complexity of the presented infection underscore the necessity for a thorough investigation into potential underlying risk factors.
The pathogenesis of fungal brain parenchymal infections, including brain abscesses, can arise either through contiguous spread from nearby sites such as sinusitis or via hematogenous dissemination from distant infections, which may be relevant to the invasive CNS (Central Nervous System) aspergillosis observed in our patient. A literature review by Ma Y et al. found 34 cases of CNS aspergillosis in immunocompetent patients. The study found that the most common cause was secondary infection following nasal sinus colonization and identified diabetes or unregulated blood sugar as a shared risk factor in 16.6% of cases [1]. While this patient did not have identified colonization of his nasal sinus, his concomitant Pseudomonas sinusitis may have provided a route of entry for the invasive fungus. A case series of 19 patients with intracranial abscesses found bacterial sinusitis to be the cause; however, in these patients the intracranial organism was usually anaerobic bacteria [2].
In addition, COVID-19 has also been associated with invasive mold infections. Moreover, his slowly worsening symptoms over a one-year period did follow an otherwise mild episode of COVID-19. However, COVID-19-associated fungal infections (CAFI) have mostly taken the form of mucormycosis, candidiasis, and pulmonary aspergillosis. The pathogenesis of CAFIs involves complex interactions among SARS-CoV-2-mediated airway epithelial damage, impaired immune responses, and dysregulation of inflammatory pathways, creating a hyperinflammatory environment that predisposes patients to secondary fungal infections like pulmonary aspergillosis [3]. This case did not involve any pulmonary components but may represent another unrecognized form of COVID-associated immune dysregulation.
Although there is no way to determine for sure, we suspect that his progressive but otherwise slow course is due to no underlying immunocompromised state. He tested negative for HIV infection, but otherwise did not receive an in-depth immunodeficiency workup.
The concept of “tissue is the issue” plays a crucial role in unraveling the complexity of this case. The subsequent return of the patient and the availability of final pathology reports unveiled the presence of Aspergillus species, emphasizing the importance of obtaining histo-pathology samples to accurately diagnose and treat complex infections. In 2018, the IDSA published guidelines for recommending the utilization of the microbiology lab in diagnosing such cases. Furthermore, they emphasized the importance of abscess aspiration and the use of the GMS stain in the histological diagnosis of these samples [4].
Surgical debridement was performed, followed by a short course of liposomal amphotericin B. Subsequently, isavuconazole was selected for ongoing treatment for several reasons. Isavuconazole is a triazole antifungal medication that inhibits the synthesis of ergosterol, a key component of fungal cell membranes, by blocking the enzyme lanosterol 14-alpha-demethylase [5]. This action disrupts fungal cell membrane integrity, leading to cell death. Isavuconazole is effective against Aspergillus species due to its broad-spectrum antifungal activity and good tissue penetration, making it a suitable treatment option for invasive aspergillosis; it is the treatment of choice in sanctuary sites such as the central nervous system [6]. It has been demonstrated in clinical trials to be non-inferior in the treatment of invasive aspergillosis to other antifungals, such as voriconazole [7] and serum levels are not required to be monitored. It differs in adverse effect profile to other azole drugs in that it causes less hepatotoxicity, as well as dose-dependent QTc shortening [5].
In conclusion, it is imperative to maintain a broad differential diagnosis. Alongside imaging findings, it is crucial to request appropriate microbiological and tissue cultures, as well as pathology and specific stains. Aggressive surgical debridement remains the cornerstone in the management of invasive fungal infections, complemented by upfront administration of liposomal amphotericin B with close monitoring. Upon achieving an appropriate clinical response, transitioning to oral therapy with isavuconazole can be considered, as demonstrated in this case.
Key References:
- Li W, Ao R, Lan X, Li Y, Zhang J, Yu S. Central nervous system aspergillosis in immunocompetent patients: Case series and literature review. Medicine (Baltimore). 2020 Oct 30;99(44). doi: 10.1097/MD.0000000000022911. PMID: 33126348; PMCID: PMC7598844.
- Maniglia AJ, Goodwin WJ, Arnold JE, Ganz E. Intracranial abscesses secondary to nasal, sinus, and orbital infections in adults and children. Arch Otolaryngol Head Neck Surg. 1989 Dec;115(12):1424-9. doi:10.1001/archotol.1989.01860360026011. PMID: 2573380.
- Hoenigl M, Seidel D, Sprute R, Cunha C, Oliverio M, Goldman GH, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022 Aug;7(8):1127-1140. doi: 10.1038/s41564-022-01172-2. Epub 2022 Aug 2. PMID: 35918423; PMCID: PMC9362108.
- Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A Guide to Utilization of the Microbiology Laboratory for Diagnosis of Infectious Diseases: 2018 Update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018 Sep 15;67(6). doi: 10.1093/cid/ciy381.
- Ellsworth M, Ostrosky-Zeichner L. Isavuconazole: Mechanism of Action, Clinical Efficacy, and Resistance. J Fungi (Basel). 2020 Nov 29;6(4):324. doi: 10.3390/jof6040324. PMID: 33260353; PMCID: PMC7712939.
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, Denning DW, Groll AH, Lagrou K, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018 May;24 Suppl 1. doi: 10.1016/j.cmi.2018.01.002. Epub 2018 Mar 12. PMID: 29544767.
- Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016 Feb 20;387(10020):760-9. doi: 10.1016/S0140-6736(15)01159-9. Epub 2015 Dec 10. PMID: 26684607.
