Title: Survival of bilateralPulmonary Mucormycosis
Submitted by: Julia Nacov, MD; Jannik Stemler, MD; Khosro Hekmat, MD; Michaela Simon, MD; Rosanne Sprute, MD; Sibylle Mellinghoff, MD; Thomas Schömig, MD; Oliver A. Cornely, MD
Institution: University Hospital Cologne
Email: julia.nacov@uk-koeln.de
Date Submitted: 01/05/2023
History:
A 47-year-old man with suspected acute leukemia due to leukocytosis, thrombocytopenia, fatigue and night sweats was transferred from an outside hospital to our Department of Hematology for further diagnostics and treatment.
After diagnosis of FLT3-TKD-, IDH2– NRAS-, KMT2A-PTD- and SRSF2-mutated acute myeloid leukemia (AML), the patient received remission-induction chemotherapy with “3+7” plus midostaurin. He obtained anti-infective prophylaxis with TMP/SMX (800/160mg, every 3 days), valaciclovir (500mg BID) and posaconazole (300mg tablets QD).
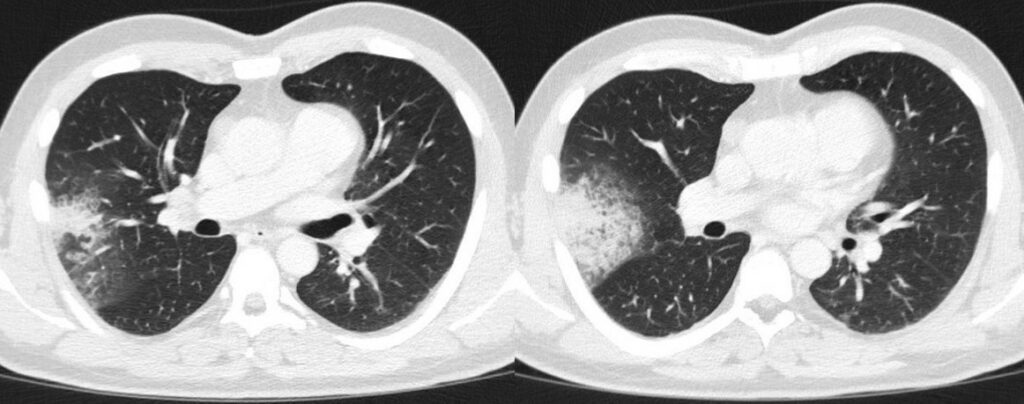
Upon admission the patient already presented with symptoms of rhinitis, fatigue, dry cough, and a sore throat. There was no evidence of respiratory pathogens in the initial virological or microbiological assessment. Baseline chest CT revealed a right upper lobe consolidation (Figure 1). Clinically the patient was persistently neutropenic and febrile despite empirical broad-spectrum antibiotic therapy with piperacillin-tazobactam (4g/0.5g QID), clarithromycin (500mg TID), and later meropenem (1g TID).
However, follow-up (FU) chest CT scans showed progressive pulmonary consolidations in both upper and lower lobes (Figure 2). Candida spp. was detected sporadically in bronchoalveolar lavage fluid, which was performed on day 8 from start of 7+3 chemotherapy. Empiric antifungal therapy was not initiated as the patient was receiving posaconazole prophylaxis from day one of chemotherapy.
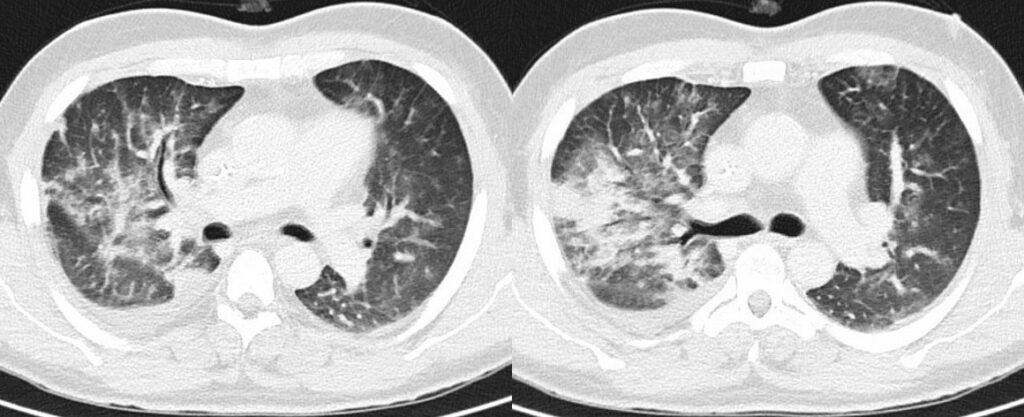
Hemophagocytic lymphohistiocytosis (HLH) was suspected as differential diagnosis due to persistent fever without pathogen detection, CT findings (Figure 3), including progressive hepatosplenomegaly, as well as high inflammatory markers (H-Score: 192 points; 80-88% probability for HLH). The patient therefore received dexamethasone 6 mg QID for five days, followed by 6 mg TID for further three days.
After initial improvement of symptoms, i.e., cough, fever, and general condition, as well as CT image morphology (Figure 4), fever recurred on day 17 from start of 7+3 chemotherapy. Furthermore, blood levels of C-reactive protein (CRP) and procalcitonin (PCT) were increased after previous decrease. Empirical caspofungin (70mg QD) treatment replaced posaconazole prophylaxis. The patient defervesced for two days, then fever recurred. Cough persisted. Intermitted oxygen therapy was needed.
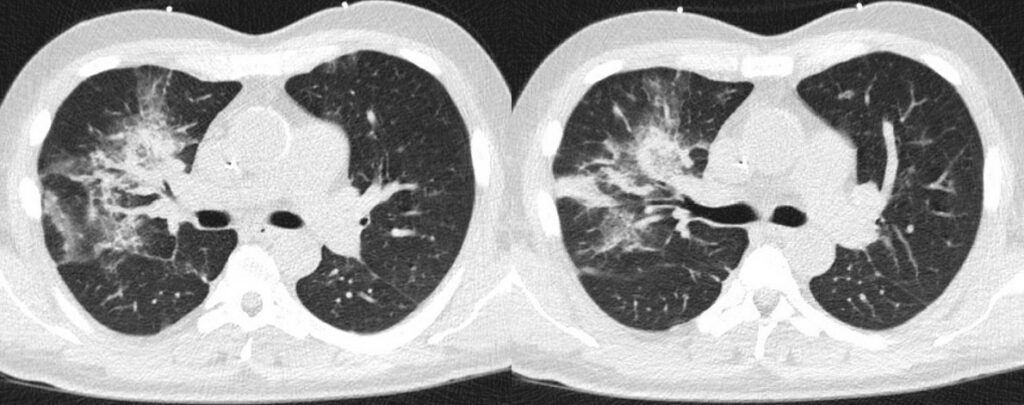
- Chest CT, axial lung window (day -1 from start of 7+3 chemotherapy): Pulmonary consolidation with air bronchograms in the right upper lobe.
- Chest CT, axial lung window (day 3 from start of 7+3 chemotherapy): Progressive pulmonary consolidation in the right upper lobe and multiple new nodular consolidations affecting all lobes. Small amounts of right pleural effusion
- Chest CT, axial lung window (day 9 from start of 7+3 chemotherapy): Progressive diffuse ground glass opacities under resolution of pulmonary consolidation in the right upper lobe. Progressive right pleural effusion.
- Chest CT, axial lung window (day 17 from start of 7+3 chemotherapy): Transverse CT of the thorax: Improving pulmonary changes with increasing consolidation of the ground glass opacities. Regressive pleural effusion.
Past Medical History:
- Root/ascending aortic aneurysm
- Aortic valve replacement (Ozaki procedure)
- Aortic root replacement (Yacoub technique)
- Anal fissure
Physical Examination:
Age: 47 years; weight: 93.5 kg; height: 178 cm.
Vital signs: Temperature: 38.8°C; BP: 100/70 mmHg; pulse: 100/min; RR: 19/min.
HEENT: Oral cavity without signs of irritation or inflammation. Right eye with subconjunctival hemorrhage. Moist mucous membranes. Axillary, cervical, supraclavicular and inguinal lymph nodes palpable.
Cardiovascular: Rhythmic, no murmurs, rubs or gallops. No signs of peripheral edema.
Respiratory: Bilaterally clear to percussion and auscultation.
Gastrointestinal: Bowel sounds present, nontender, nondistended, soft, no masses.
Neurologic: No meningism, isochoric pupils, pupillary light reflex normal, without focal neurological deficit, fully oriented.
Laboratory Examination (range):
– White Blood Count (WBC): 16.72 x1E9/l (ref. 4.4 – 11.3)
– Red Blood Count (RBC): 2.25 x1E12/l (ref. 4.5 – 5.9)
– Hemoglobin: 6.6 g/dl (ref. 13.50 – 18.0)
– Hematocrit: 20 % (ref. 42 – 50)
– Platelets (PLT): 610 x1E9/l (ref. 150 – 400)
– CRP: 151.6 mg/l (ref. <5,0)
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed:
- Blood cultures: negative for bacteria and fungi
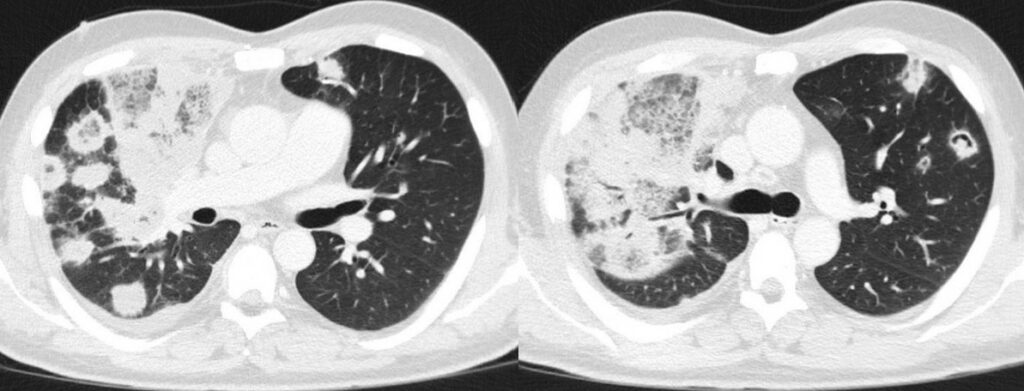
- Chest CT: ground-glass opacities, consolidations with reversed halo sign, cavities, pleural effusion.
- Abdominal ultrasound: splenomegaly and subcapsular low-echo lesion.
- Repeated Bronchoalveolar lavage fluid (BALF):
- Positive Mucorales spp. PCR (2 independent assessments).
- Positive culture with E. faecium (moderate amount).
Transverse Computed Tomography (CT) of the thorax:
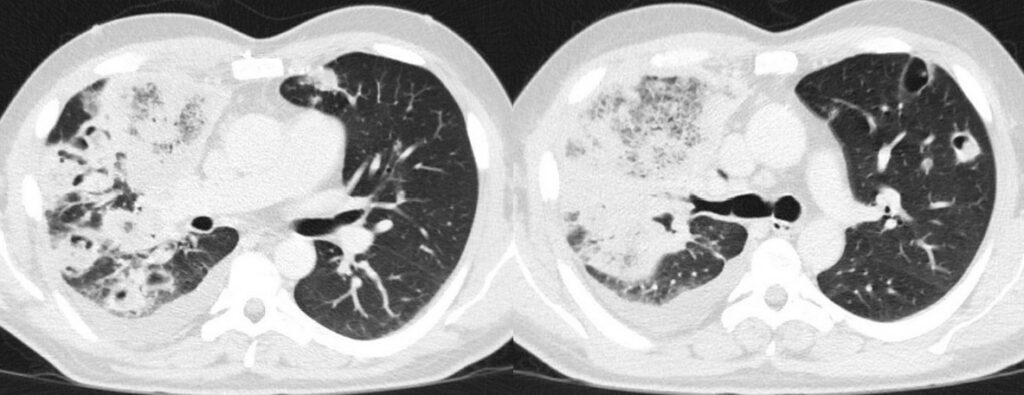
- Chest CT, axial lung window (day 28 from start of 7+3 chemotherapy): Massive progressive bilateral pulmonary consolidations with ground glass opacities and partly reversed halo sign. Implied starting cavitation and recurred right pleural effusion.
Probable Invasive Pulmonary Mold Diseases (EORTC/MSG ERC criteria1):
- Host factors:
- Recent history of neutropenia temporally related to the onset of invasive fungal disease: <0.5 × 109 neutrophils/L for >10 days
- Hematologic malignancy: AML
- Clinical features:
- The presence of the following patterns on CT:
- Dense, well-circumscribed lesions with or without a halo sign
- Air crescent sign
- Cavity
- Reverse halo sign
- The presence of the following patterns on CT:
- Mycological evidence: EORTC/MSG ERC criteria not met before surgery
Clinical course:
Chest CT displayed findings suspicious of pulmonary mucormycosis, which was then confirmed by a positive polymerase chain reaction (PCR) test for Rhizomucor spp. on bronchoalveolar lavage fluid (BALF). BALF culture yielded growth of E. faecium.The culture yielded no fungal growth. Additionally, abdominal ultrasound revealed splenomegaly and a subcapsular low-echo lesion suspicious of splenic infarction, possibly due to mucormycosis.
Final Diagnosis: Pulmonary mucormycosis
Question 2: What would be the recommended treatment?
Treatment:
The recent Global Guideline for the Diagnosis and Management of Mucormycosis strongly recommends first line antifungal monotherapy with liposomal amphotericin B across all patterns of organ involvement2. In case of nephrotoxicity, dose may be reduced, but doses below 5mg/kg QD are supported with marginal strength only2. Combination treatment with isavuconazole or posaconazole may be considered in rapidly progressing or extensive disease, as well as reduced overall condition2,3. Supplementary surgical treatment is strongly advised whenever feasible2. Continuation of treatment until resolution of related CT findings and restoration of patient’s immune system, as well as the use of isavuconazole in case of switching to oral medication, is strongly supported by the guideline group2.
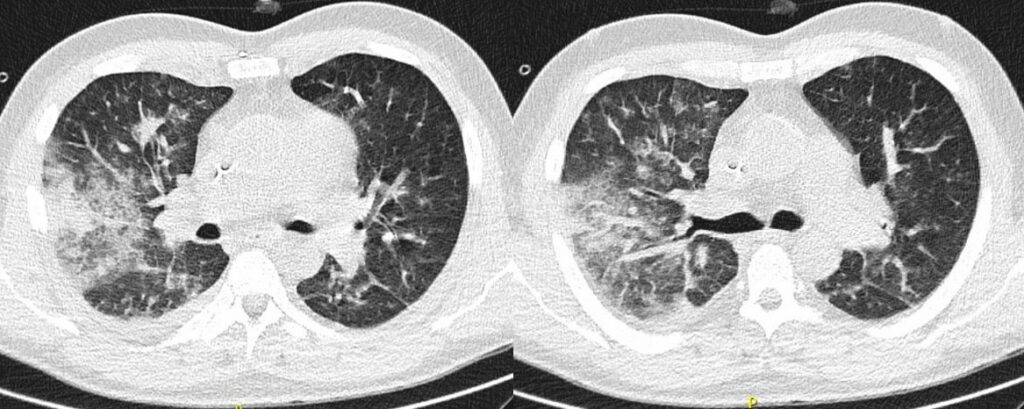
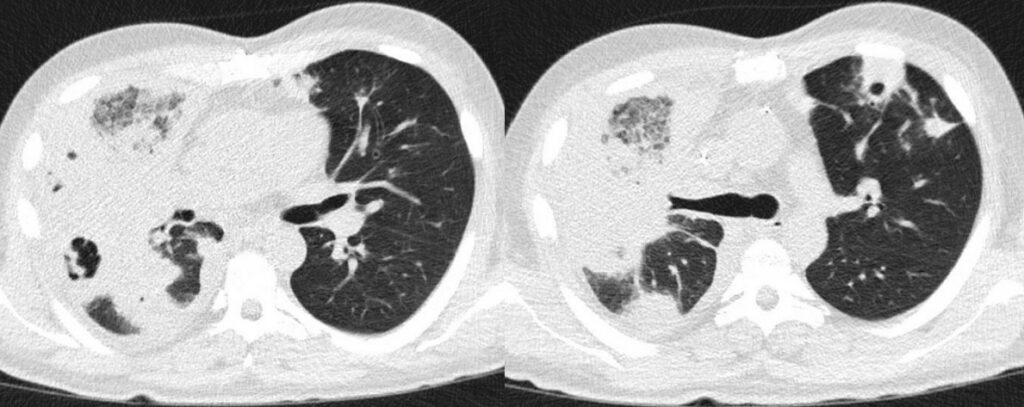
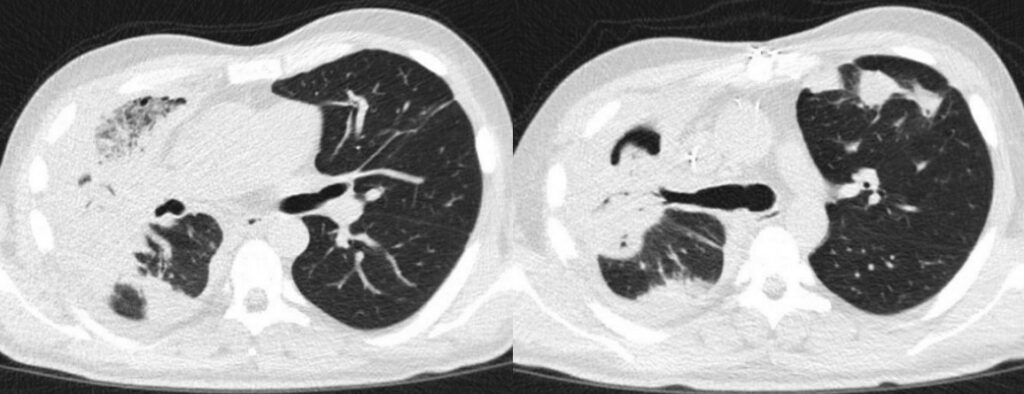
Besides broad-spectrum antibiotic therapy (meropenem 1g TID), the patient received high-dose liposomal amphotericin B (10 mg/kg QD) intravenously, which was reduced to 8 mg/kg QD after two days, and finally to 5 mg/kg QD after another 10 days because of deteriorating renal function. Antifungal treatment was supplemented by isavuconazole (loading dose, followed by 200mg QD) after first amphotericin B dose reduction, and finally continued as monotherapy after 46 days of combination therapy. FU chest CT scans were performed every 7 to 14 days (Figure 6-13). After initial progress in the first two FUs, the CT findings remained stable with bilateral findings, but right-sided accentuation. In the further clinical course, the patient developed recurrent hemoptysis, and was monitored on the intermediate care unit (IMC) due to high-risk of pulmonary bleeding. After two months of antifungal treatment and slight improvement of lesions in the left lung, he was transferred to our cardio-thoracic surgery for right sided pneumonectomy.
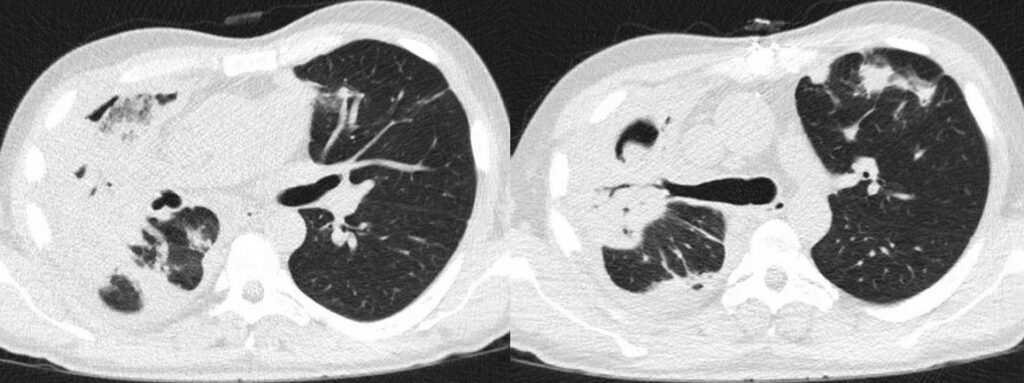
- Chest CT, axial lung window: 1st FU (7 days): Further progressive bilateral consolidations as well as progressive cavitation and right pleural effusion.
- Chest CT, axial lung window: 2nd FU (14 days): Progressive fusion of the consolidations in the right upper lobe. Slightly decreasing pleural effusion.
- 8.Chest CT, axial lung window: 3rd FU (21 days): Stable findings.
- Chest CT, axial lung window: 4th FU (28 days): Stable findings.
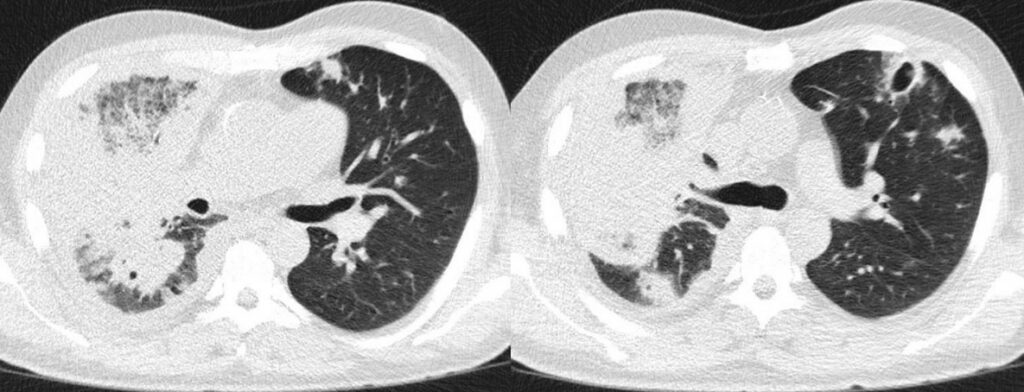
- Chest CT, axial lung window: 5th FU (35 days): Stable findings.
- Chest CT, axial lung window: 6th FU (42 days): Stable findings.
- Chest CT, axial lung window: 7th FU (49 days): Stable findings.
- Chest CT, axial lung window: 8th FU (56 days): Slightly shrinking bilateral consolidations and regressive right pleural effusion.
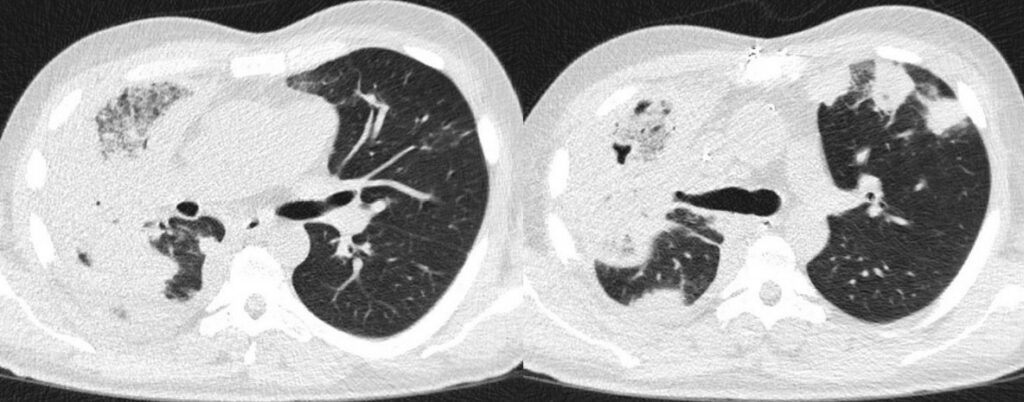
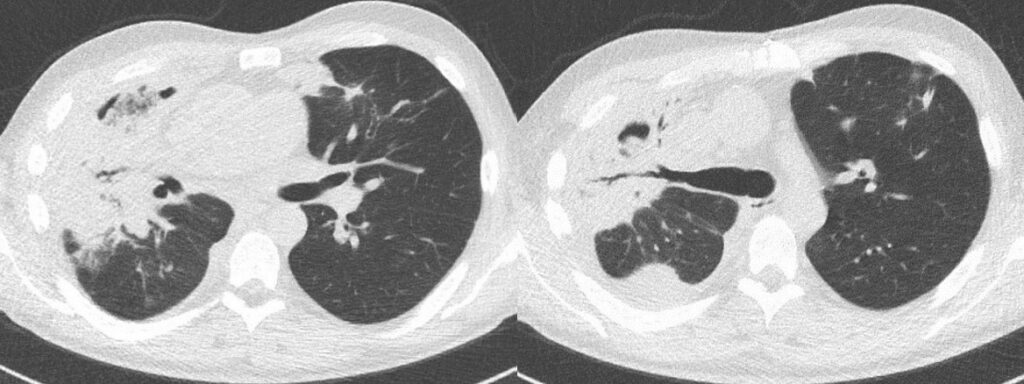
Outcome:
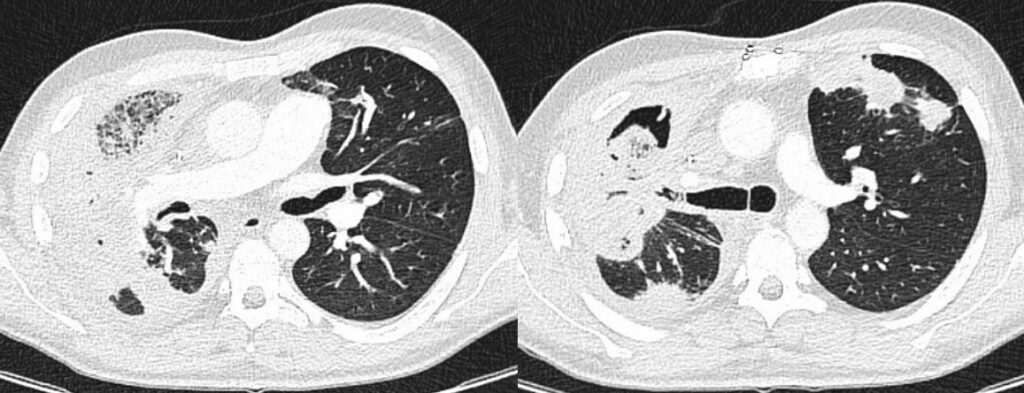
The surgical procedure went without complications. Tissue samples and swabs including exact anatomical location for microbiology and pathology lab were collected during surgery according to the local European Confederation of Medical Mycology (ECMM) emergency procedure pathway for suspected invasive mucormycosis4. Species identification could therefore be achieved (see below). EORTC/MSG ERC criteria for proven invasive mold disease1 were consequently met (see below). The patient was transferred back to our Department of Hematology 14 days after surgery for further treatment. Repeated CT scans showed steady improvement of residual consolidations (Figure 14-15). Antifungal treatment with isavuconazole was continued after surgical treatment until resolution of CT findings and immunological restoration. The patient was discharged from the hospital on day 119 day 17 from start of 7+3 chemotherapy and is followed in an outpatient clinic. With regard to AML, an allogeneic stem cell transplant is planned.
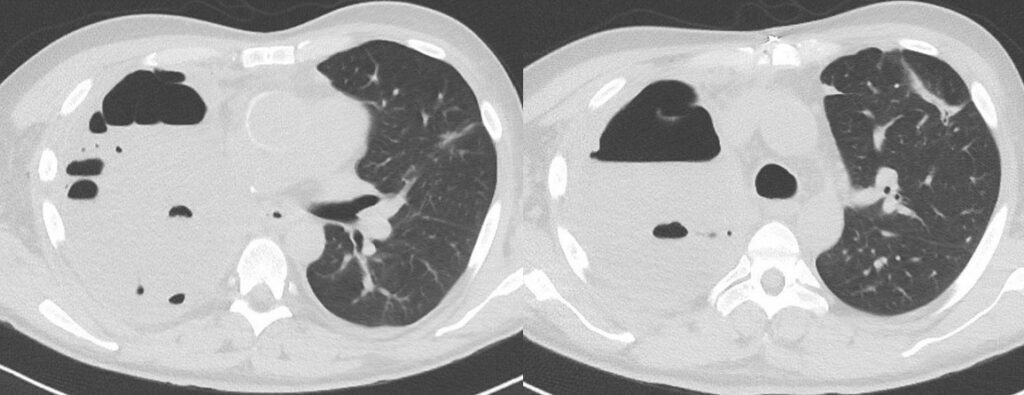
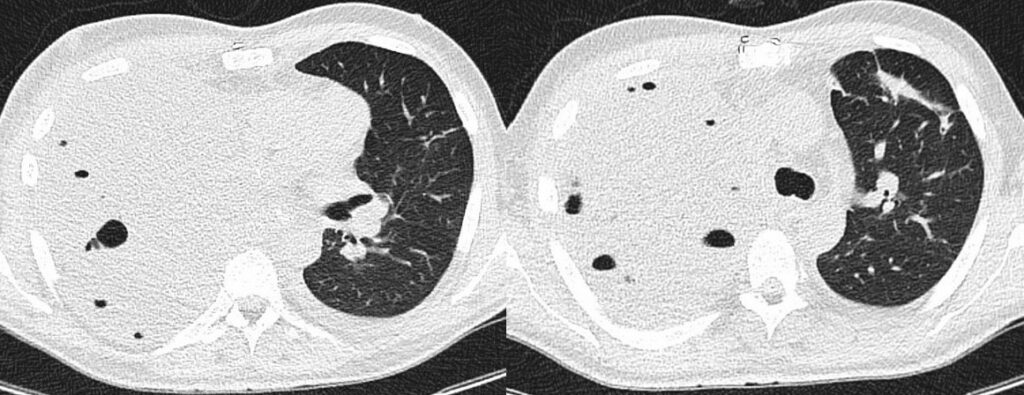
- Chest CT, axial lung window: 1st FU after surgery (10 days): Both liquid- and air-filled cavity right after pneumectomy. Streaky scarred pulmonary changes in position further consolidations in the left upper lobe.
- Chest CT, axial lung window: 2nd FU after surgery (18 days): Progressive liquid-filled cavity after pneumectomy. No further changes.
Microbiology (intraoperative samples):
- Smear right upper lobe: Positive culture with E. faecium
- Biopsy right upper lobe (removed during surgery):
- Positive culture with E. faecium
- Molecular identification: Rhizomucor pusillus (species identification by large subunit (LSU) ribosomal deoxyribonucleic acid (rDNA) D1-D2 sequences)
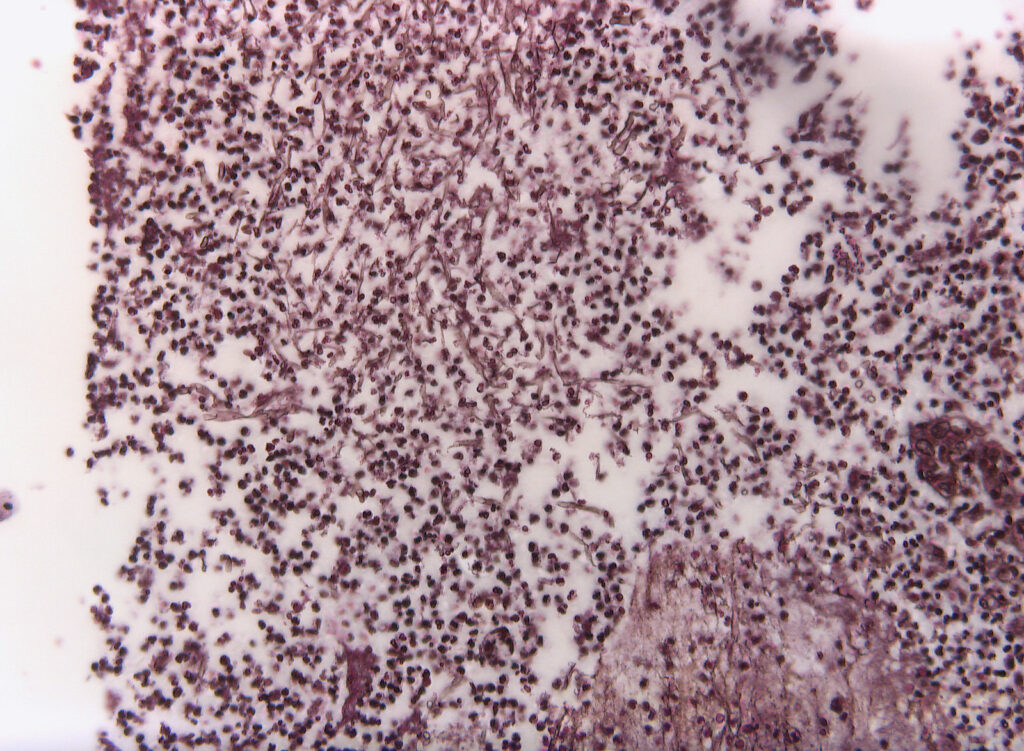
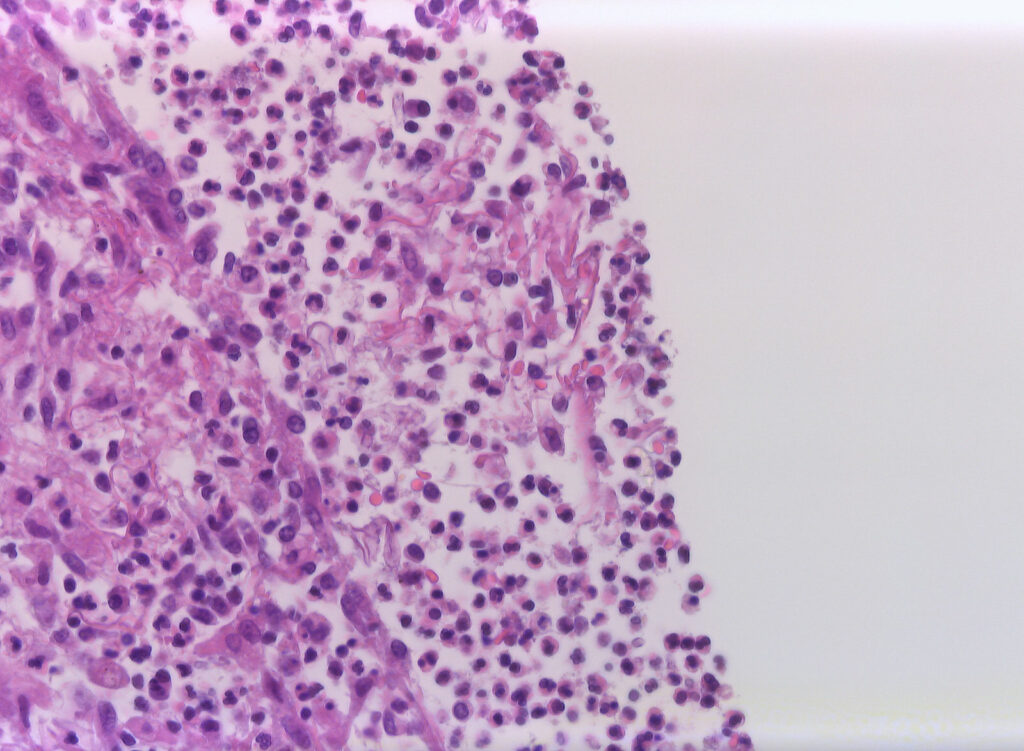
Histopathology (intraoperative samples):
- Biopsy right upper lobe (removed during surgery): extensive purulent-abscessing bronchopneumonia and purulent pleurisy. Detection of 6-7µm wide, irregular fungal hyphae with right-angle branching and rare septation.
- Biopsy right: Grocott-Gomori methenamine silver (GMS) stain: Fungal hyphae
- Biopsy right: Hematoxylin-Eosin (HE) stain: Fungal hyphae
Proven Invasive Pulmonary Mold Diseases (EORTC/MSG ERC criteria1):
- Microscopic Analysis: Sterile Material
- Histopathologic, cytopathologic, or direct microscopic examination of a specimen obtained by needle aspiration or biopsy in which hyphae or melanized yeast-like forms are seen accompanied by evidence of associated tissue damage
- Tissue Nucleic Acid Diagnosis
- Amplification of fungal DNA by PCR combined with DNA sequencing when molds are seen in formalin-fixed paraffin-embedded tissue
Discussion: (682 words)
Pulmonary mucormycosis is a life-threatening fungal infection with rare occurrence and high morbidity, mostly affecting immunocompromised patients with underlying hematological malignancy and profound neutropenia5,6. Mortality is high, ranging up to 76%5. Clinical manifestation varies, but prolonged fever is seen in most patients7. Rapid diagnosis and intervention are crucial due to fast and aggressive disease progression8. Delayed initiation of therapy is related with increased mortality8.
Diagnostic tools to detect pulmonary mucormycosis include imaging (i.e., Chest CT), histopathology, direct microscopy, and culture, as well as molecular-based tests2. Microbiological testing can be performed on BAL and/or biopsy specimen9. Baseline chest computed tomography (BCT) in high-risk hematology patients allows early diagnosis and has therefore become standard of care at numerous hematology departments10,11. In case of a reversed halo sign on chest CT, radiographic staging of head, neck and abdomen should be initiated12. To verify the adherence to current guideline recommendations in the management of mucormycosis the EQUAL Mucormycosis Score can be used9.
In our case, multiple chest CT scans were performed throughout the clinical course. While the baseline chest CT on admission displayed right upper lobe consolidation (Figure 1), suspicious for lobar pneumonia, pulmonary lesions evolved from bilateral consolidations to atypical infiltrates (Figure 2-4) and finally findings suspicious of pulmonary mucormycosis (e.g., reversed halo, Figure 5) on day 28 from chemotherapy initiation during neutropenia. Considering the timeline of events, treatment with high-dose corticosteroids due to initially suspected HLH must be taken into account as a risk factor for subsequent fungal infection1. Radiographic staging additionally revealed splenomegaly and a subcapsular lesion suspicious of splenic infarction or mucormycosis.
BALF was twice obtained for microbiological testing. E. faecium was isolated from two distinct samples. Its pathological relevance is questionable, as not commonly known to cause pneumonia13. Both, direct microscopy, and culture were consistently negative for other pathogens including fungi. Therefore, antifungal susceptibility testing was not performed. Sensitivity of culture was shown to be limited with only one third of all microscopically positive specimens yielding positive cultures14. In our case, detection of Mucorales spp. was achieved using Mucorales-PCR on BALF, and species identification (i.e., Rhizomucor pusillus) by analyses of D1-D2 regions of large subunit ribosomal deoxyribonucleic acid (rDNA) sequences on biopsy specimen from surgery later in the clinical course. Molecular based tests, thus, represent major microbiological diagnostic tools, contributing to fungal detection, as well as species identification in the absence of cultural growth and negative microscopy. Regarding the latter, direct microscopic examination of specimens obtained from surgery later in the clinical course finally revealed fungal hyphae and associated tissue damage. While width of hyphae was rather in the lower range pathognomonic for mucormycosis (i.e., 6-7 µm), possibly due to long-duration of previous antifungal therapy or altered appearance after fixation. Morphology was otherwise typical with irregular, right-angle branching and rare septation15. Hence, two EORTC/MSG ERC criteria for proven invasive pulmonary mold diseases (i.e., microscopic analysis and tissue nucleic acid diagnosis) were ultimately met1.
Once mucormycosis was suspected in our patient, drug treatment with high-dose liposomal amphotericin B was immediately started as strongly recommended by the ECMM global guideline2. Due to deteriorating renal function dose was adjusted and successively reduced, but not below 5 mg/kg QD as recommended by the guideline group. Although there is only limited data supporting combination therapy, which is therefore supported by the guideline group with marginal recommendation only, additional treatment with isavuconazole or posaconazole may be considered in terms of rapidly progressing or extensive disease2,3. With consolidations in both lungs and therefore limited options for initial surgical treatment, isavuconazole was supplemented after liposomal amphotericin B dose reduction and finally continued as monotherapy as stated above. The splenic lesion suspicious for splenic infarction or mucormycosis successively improved during antifungal treatment.
According to the global guideline for mucormycosis, surgical treatment should be performed whenever possible2. Finally, after two months of antifungal treatment and improvement of lesions in the left lung (Figure 12-13), the patient was transferred to cardio-thoracic surgery.
While pulmonary mucormycosis remains a severe and aggressive fungal infection with extremely high morality in immunocompromised hosts, our patient survived despite bilateral lung involvement as well as delayed surgical treatment.
Key References:
1. Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 2020; 71(6): 1367-76.
2. Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis 2019; 19(12): e405-e21.
3. Vehreschild JJ, Birtel A, Vehreschild MJ, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol 2013; 39(3): 310-24.
4. Stemler J, Koehler P, Cornely OA. European Confederation of Medical Mycology Diamond Excellence Center suspected invasive mucormycosis – emergency procedure. PUBLISSO: Universitätsklinikum Köln. Klinik I für Innere Medizin; 2022.
5. Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis 2005; 41(5): 634-53.
6. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev 2000; 13(2): 236-301.
7. Pagano L, Ricci P, Tonso A, et al. Mucormycosis in patients with haematological malignancies: a retrospective clinical study of 37 cases. GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto). Br J Haematol 1997; 99(2): 331-6.
8. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis 2008; 47(4): 503-9.
9. Koehler P, Mellinghoff SC, Lagrou K, et al. Development and validation of the European QUALity (EQUAL) score for mucormycosis management in haematology. J Antimicrob Chemother 2019; 74(6): 1704-12.
10. Stemler J, Bruns C, Mellinghoff SC, et al. Baseline Chest Computed Tomography as Standard of Care in High-Risk Hematology Patients. J Fungi (Basel) 2020; 6(1).
11. Bitterman R, Hardak E, Raines M, et al. Baseline Chest Computed Tomography for Early Diagnosis of Invasive Pulmonary Aspergillosis in Hemato-oncological Patients: A Prospective Cohort Study. Clinical Infectious Diseases 2019; 69(10): 1805-8.
12. Cornely OA, Arikan-Akdagli S, Dannaoui E, et al. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect 2014; 20 Suppl 3: 5-26.
13. Li F, Wang Y, Sun L, Wang X. Vancomycin-resistant Enterococcus faecium pneumonia in a uremic patient on hemodialysis: a case report and review of the literature. BMC Infectious Diseases 2020; 20(1): 167.
14. Lackner M, Caramalho R, Lass-Flörl C. Laboratory diagnosis of mucormycosis: current status and future perspectives. Future Microbiol 2014; 9(5): 683-95.
15. Challa S. Mucormycosis: Pathogenesis and Pathology. Current Fungal Infection Reports 2019; 13(1): 11-20.
