Submitted by: Sarah Tran MD, Caroline Hamilton PA
Institution: Augusta University
Email: satran@augusta.edu
Date: 5/24/21
History:
Chief Complaint: Increasing shortness of breath in a patient recently diagnosed with COVID-19.
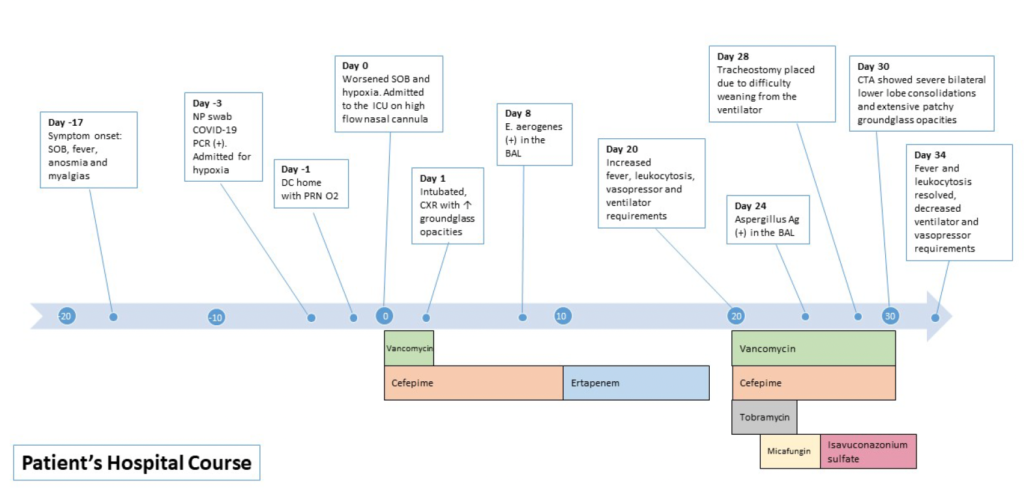
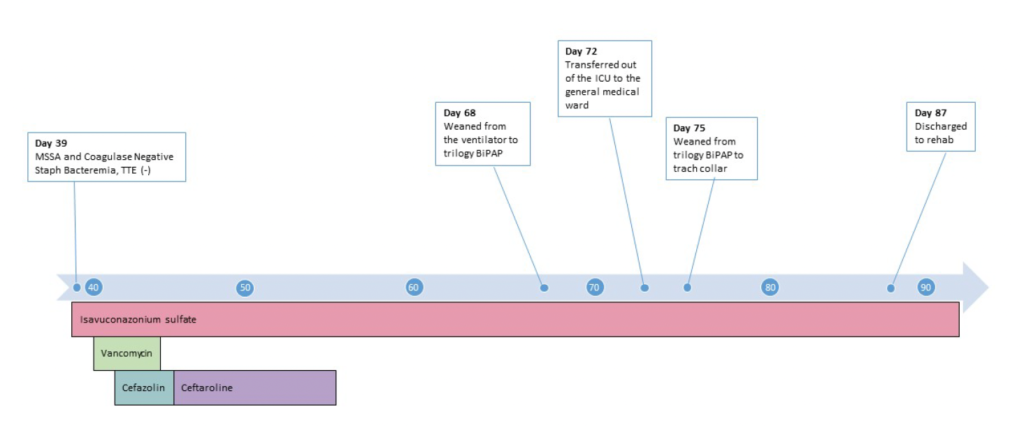
66-year-old white male admitted to the hospital for COVID-19 pneumonia with a two-week history of worsening shortness of breath, fevers, myalgias, anosmia, nausea and poor oral intake. He received dexamethasone and tocilizumab and was discharged home in a stable condition. Within 24 hours of discharge, he was readmitted with worsening tachypnea and hypoxia. He was placed on high flow nasal cannula initially but ultimately required intubated and admission to the ICU. While he did not meet criteria for remdesivir, he received convalescent plasma and continued on dexamethasone. The patient had a prolonged ICU stay of greater than one month complicated by mechanical ventilation dependence due to hypoxemic respiratory failure, persistent fevers with leukocytosis, and MSSA bacteremia. He then developed acute worsening of hypoxia and leukocytosis and was treated for presumed Enterobacter ventilator-associated pneumonia without clinical improvement. Additional laboratory specimens and diagnostic tests were requested.
Medical Hx
Obstructive sleep apnea on CPAP
Obesity (BMI 35.1 kg/m2)
Hypertension
Hyperlipidemia
Family Hx
Mother: Hypertension
Sister: Diabetes
Brother: Hypertension
Surgical Hx
None
Review of Symptoms:
Present
Shortness of breath
Productive cough
Generalized weakness
Not Present
Chest pain, palpitations
Fevers, chills, headaches
Nausea, vomiting, diarrhea, constipation
Numbness, tingling
Rash, skin lesions
Dysuria, hematuria
Myalgias, arthralgias
MEDICATIONS – at admission
Lisinopril 40mg daily
Rosuvastatin 20mg daily
Pantoprazole 40mg daily
Physical Examination:
General: Alert, awake. Well-developed, well-nourished male in mild respiratory distress.
Skin: Warm, dry, no rashes over trunk and extremities.
Head: Normocephalic, atraumatic.
Eye: EOMI, normal conjunctiva. PERRL.
Ears, nose, mouth and throat: Oral mucosa moist, No oropharyngeal lesions identified.
Cardiovascular: Regular rate, Regular rhythm. No murmurs, rubs or gallops. Well-perfused extremities with 2+ distal pulses. No peripheral edema noted.
Respiratory: Lungs clear bilaterally. Increased work of breathing, on high flow nasal cannula.
Gastrointestinal: Normoactive bowel sounds. Soft, non-distended, non-tender to palpation.
Musculoskeletal: Normal muscle bulk and tone. No gross deformity.
Neurological: Alert and oriented to person, place, time, and situation, No gross focal neurological deficit.
Psychiatric: Cooperative, anxious and tearful.
Admission Labs:
CHEMISTRY/METABOLIC PANEL
Na: 136 mEq/L
K: 4.5 mEq/L
Cl: 102 mEq/L
CO2: 24 mEq/L
BUN: 23 mg/dL
SCr: 1.19 mg/dL
Glucose: 122 mg/dL
Calcium: 8.5mg/dL
Total protein: 7.1 g/dL
Albumin: 3.7 g/dL
AST: 47 U/L
ALT: 27 U/L
Alk Phos: 66 U/L
T bili: 0.3 mg/dL
CBC
WBC: 16.8 thous/mm3
RBC: 4.96 million/mm3
HGB: 10.9 g/dL
HCT: 35.3%
MCV: 22.5 pg
MCH: 32.1 g/dL
RDW: 19.1%
PLT: 323 thous/mm3
Other labs
Troponin I: <0.010 ng/mL
BNP: 19.0 pg/mL
Ferritin: 118.2 ng/mL
CRP: 1.060 mg/dL
What Are Probable/Possible Diagnoses?
– Post COVID Inflammatory Syndrome
– Ventilator-Associated Pneumonia
– Invasive Fungal Infection
– Pulmonary MAC
– Pneumocystis Pneumonia
Microbiology/Diagnostic Tests Performed:
• BAL Bacterial Cx: Klebsiella (Enterobacter) aerogenes
• BAL KOH smear: Negative
• BAL Fungal Cx: No growth
• BAL Galactomannan: 4.721 (normal < 0.500)
• Serum Aspergillus Ag: 0.156 (normal < 0.500)
• Serum B-D Glucan: < 31 (normal)
• Legionella Ag: Negative
CTA Chest: Severe bilateral lower lobe consolidations with scattered small consolidations in the remaining lobes. Extensive patchy ground glass opacities and septal thickening throughout all lobes. Small bilateral pleural effusions. Atelectatic changes in the dependent lungs.
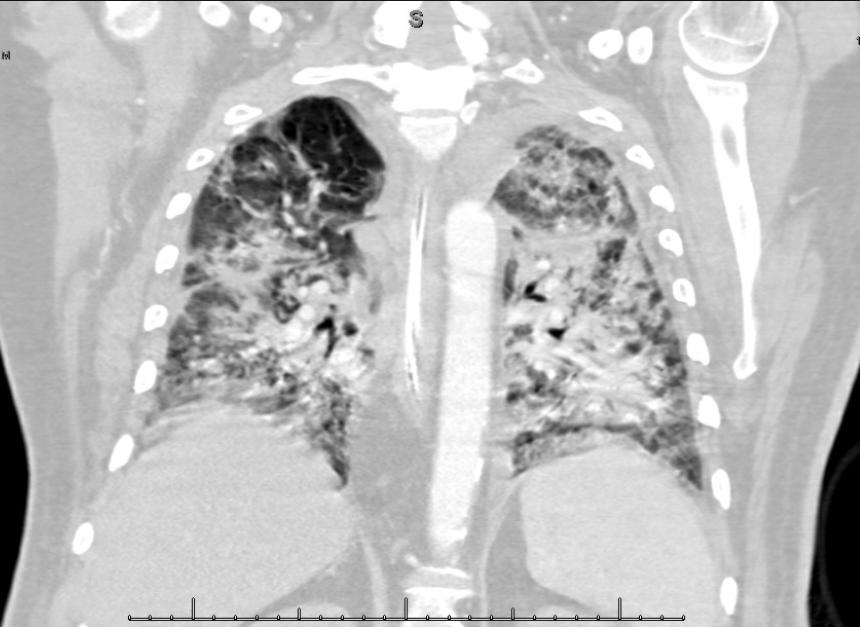
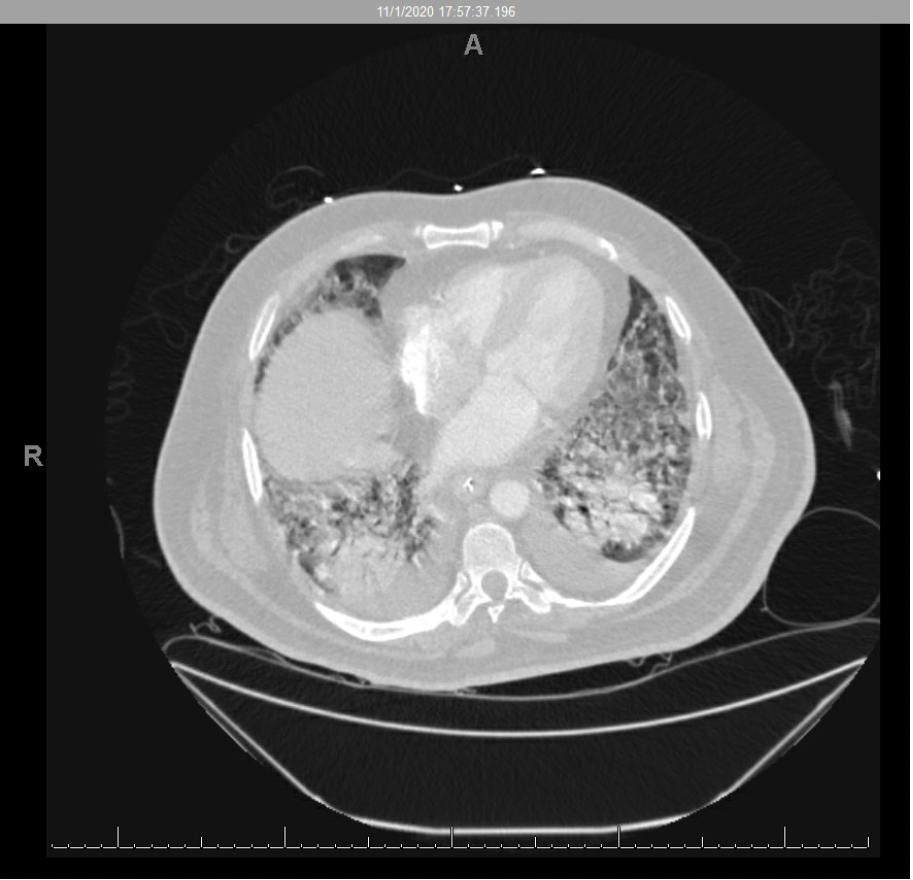
Timeline of Hospital Course / Images
WHAT DID YOU FIND MOST CHALLENGING ABOUT THIS CASE?
The most challenging part of this case was the sheer complexity of the patient’s hospital course and his nontraditional risk factors for invasive fungal disease. Prior to being diagnosed with COVID-19 pneumonia, he did not have any significant immunosuppression that would routinely predispose him to invasive fungal disease. Additionally, he had a very protracted hospital course secondary to his refractory hypoxemia, with numerous bacterial complications including a recently diagnosed Enterobacter aerogenes pneumonia. Despite what should have been adequate treatment for the bacterial pneumonia, his fevers persisted. While continued fevers can be associated with severe COVID-19 disease alone, in this patient’s case, it was a marker of yet unidentified underlying fungal disease.
As such, the diagnosis of COVID-19 associated pulmonary aspergillosis in this case required providers to maintain a broad differential and to be mindful of the specific risk that COVID-19 carries for development of invasive mold infections.
WHAT DO YOU SEE AS THE BIGGEST UNMET EDUCATIONAL NEED IN MANAGING COVID-19-ASSOCIATED INVASIVE FUNGAL INFECTIONS?
Over the course of the COVID-19 pandemic, Invasive Pulmonary Aspergillosis (IPA) has been increasingly identified in otherwise immunocompetent patients admitted with COVID-19 pneumonia worldwide, termed COVID-19-Associated Pulmonary Aspergillosis (CAPA). Prior to the pandemic, the concern for IPA would usually be low in a previously immunocompetent patient. Cases of invasive pulmonary aspergillosis are increasingly being identified in patients admitted with COVID-19 with no preceding immunocompromising conditions. This may be attributed to the immunosuppressing drugs used for COVID-19 or an inherent characteristic of COVID-19 disease itself. It is not yet clear what subset of the population admitted with COVID-19 is at highest risk of developing IPA, especially given variable reported incidence rates. This is a major opportunity to educate providers to ensure that IPA is maintained on the differential in patients with COVID-19 disease, as it has high mortality within the critically ill population and requires clinicians to be timely in its diagnosis and treatment. Similarly, given its relatively novel nature, there are no strict guideline recommendations for the optimal duration of therapy. As such, providers will need to closely monitor patients for the duration of treatment and tailor therapy based on the individual patient’s clinical improvement and any ongoing immunosuppression.
