Title: A Fungal Cause of Neutropenic Fever
Submitted by: Adam Ressler, MD; Megan Neils, MD; Laraine Washer, MD; Kathleen Linder, MD
Institution: University of Michigan
Email: resslead@med.umich.edu
Date Submitted: 11/23/2020
History:
A 48-year-old female with type 2 diabetes mellitus and stage IV diffuse large B cell lymphoma complicated by secondary hemophagocytic lymphohistiocytosis (HLH) was admitted to a tertiary care center for management of neutropenic fever.
One month prior to this admission, the patient was hospitalized at our center with thrombocytopenia (platelet count 32 x103/µL) and altered mental status. Preliminary bone marrow biopsy showed markedly hypercellular marrow with a large CD20 population. Peripheral blood flow cytometric immunophenotyping was concerning for a mature B cell lymphoma and subsequent bone marrow biopsy confirmed stage IV diffuse large B cell lymphoma. She was initiated on chemotherapy with R-CHOP and high dose methotrexate (3.5 g/m2). Her course was complicated by HLH, as defined by the HLH-2004 criteria[1] (presence of splenomegaly, fever, hypertriglyceridemia, ferritin >500 mcg/L, cytopenias, and elevated soluble IL-2 receptor). HLH was felt to be secondary to her lymphoma, as there was no infectious trigger identified. She was continued on high dose steroids (initially dexamethasone 20 mg daily) with a taper for treatment of HLH. She was discharged home from the hospital to continue chemotherapy as an outpatient.
Approximately two days after discharge, the patient presented to a local hospital with fever of 101.4°F. She noted pain over her right chest near the site of an indwelling port, intermittent abdominal pain, and mild dysuria. Laboratory evaluation demonstrated pancytopenia with white blood cell count 2.1 x103/µL (100% neutrophils), hemoglobin 6.7 g/dL, and platelet count 25 x 103/µL. Chest x-ray showed new small bilateral opacities. CT scan of her abdomen and pelvis was unremarkable. Blood cultures were obtained and the patient was started on vancomycin, cefepime, and metronidazole.
Blood cultures returned positive for yeast 24 hours after admission. She was started empirically on liposomal amphotericin B and voriconazole and was transferred to our center for continuity of care.
Physical Examination:
Physical exam demonstrated an ill-appearing adult female in no acute distress. She had a port in place on her right chest without surrounding erythema or edema. There was no palpable cervical or axillary adenopathy. Abdominal exam was unremarkable. Her skin exam was notable for shallow ulcerations associated with ruptured bullae on the medial thighs bilaterally.
Laboratory Examination:
The patient was pancytopenic with a total white blood cell count of <0.1 x103/µL, hemoglobin of 6.9 g/dL, and platelet count of 41 x103/µL. Her creatinine was 1.99 mg/dL from a baseline of 0.3 mg/dL.
Question 1: What are probable/possible diagnoses?
Because a yeast was growing from the blood culture bottles, our differential diagnosis included candidemia, disseminated cryptococcal infection, disseminated histoplasmosis or fungemia with a non-Candida yeast.
Microbiology/Diagnostic Tests Performed:
Serum cryptococcal antigen (CRAG) was positive with a Cryptococcus neoformans titer of 1:1280.
Abdominal ultrasound demonstrated cirrhotic liver morphology with sequela of portal hypertension including moderate volume ascites.
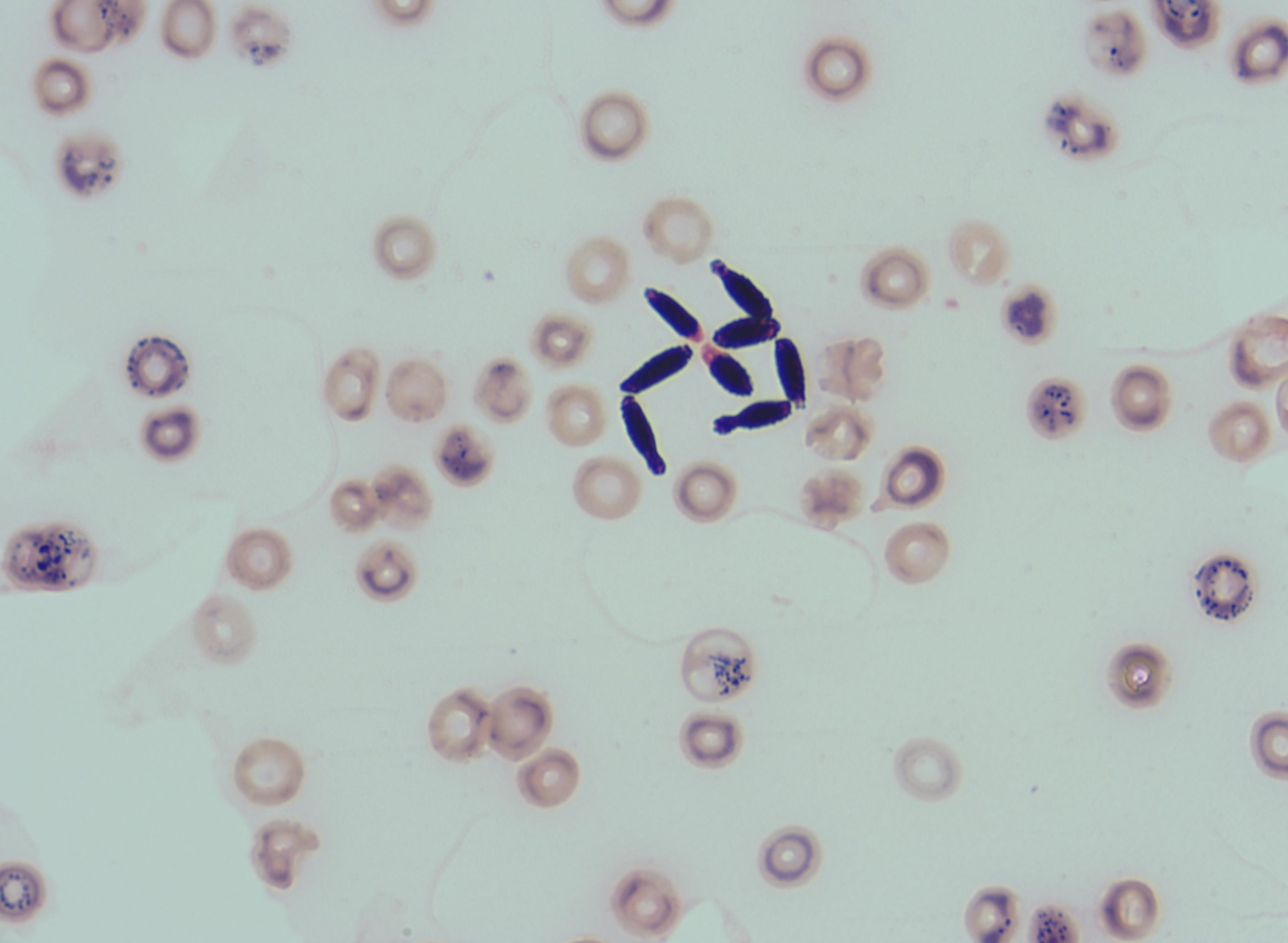
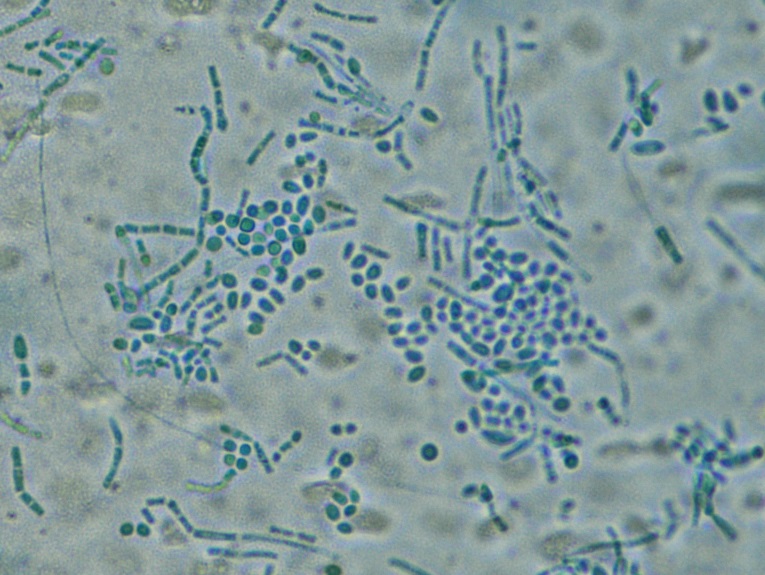
Blood cultures were repeated and remained positive for yeast. Gram stain of the patient’s blood cultures demonstrated budding yeast forms (Figure 1). Lactophenol blue preparation showed arthroconidia and yeast forms (Figure 2). This yeast was identified by MALDI-TOF as Trichosporon asahii.
Susceptibility testing demonstrated a minimum inhibitory concentration (MIC) of 1 mcg/mL for amphotericin B, > 8 mcg/mL for micafungin, 8 mcg/mL for fluconazole, 0.25 mcg/mL for itraconazole, 0.25 mcg/mL for posaconazole and 0.12 mcg/mL for voriconazole.
Final Diagnosis: Trichosporon asahii fungemia
Question 2: What treatment is recommended in the care of this patient?
Treatment:
The patient was initially started on dual antifungal therapy with liposomal amphotericin B and voriconazole. Once Trichosporon asahii was identified, liposomal amphotericin B was discontinued. The patient was continued on PO voriconazole 4 mg/kg adjusted body weight every twelve hours. Patient’s port was removed.
Despite voriconazole therapy with therapeutic levels (trough 8.7 µg/mL), the patient remained fungemic. A dilated eye exam did not show fungal endophthalmitis. Transthoracic and transesophageal echocardiograms did not demonstrate valvular vegetations. A positron emission tomography (PET) scan showed diffuse hypermetabolism within the kidney parenchyma bilaterally with otherwise physiologic uptake.
Outcome:
The patient was fungemic for 15 days. Ultimately, liposomal amphotericin B was added on day 13 of persistent fungemia with subsequent clearing of blood cultures on day 15. She had one isolated positive blood culture for T. asahii 6 days later; this cleared without change in therapy. Liposomal amphotericin B and voriconazole combination therapy was continued for 2 weeks following clearance of blood cultures and she was transitioned to PO voriconazole alone with plans for long term oral therapy.
Discussion:
Trichosporon species are non-encapsulated basidiomycetous yeasts which are commonly found in the environment and as part of the skin flora, particularly in the groin or peri-genital areas and are an uncommon cause of infections in humans[2]. When infection does occur, it is typically in the setting of severe immunocompromise, particularly in patients with hematologic malignancy and prolonged neutropenia[2]. Other underlying conditions which have been associated with Trichosporon infection risk include diabetes, pulmonary diseases and other malignant tumors[3,4]. Trichosporon asahii is the most common species causing human infection, accounting for about 35-84% of reported Trichosporon infections[2,5].
The most common manifestation of invasive Trichosporon infection is fungemia[2,5].Other presentations which have been reported include endocarditis, peritonitis, meningitis, urinary tract infection, and chronic hepatic trichosporonosis[2,3,6,7]. The most common routes of infection with Trichosporon are via indwelling vascular catheters or through the gastrointestinal tract[7,8]. A recent epidemologic study of 140 patients with Trichosporon asahii infection identified broad spectrum antibiotic use, invasive medical devices (including central venous catheters), neutropenia and intensive care unit stay as the most significant risk factors[3]. Other identified risk factors include peritoneal dialysis, corticosteroid use, and cytotoxic chemotherapy[2]. This is a rare infection; one retrospective study conducted in 15 Italian hospitals over a 20 year period only identified 17 cases of proven/probable Trichosporon infection (an incidence rate of 0.4%); 15 of whom had received cytotoxic chemotherapy and 12 had a neutrophil count of <100/mm3 at the time of infection[4].
Trichosporon spp express a glucuronoxylomannan (GXM) polysaccharide in their cell walls which is antigenically similar to that of Cryptococcus neoformans[8]. This is an important virulence factor for this organism as high concentrations of GXM can inhibit phagocytosis[8,9].This similarity also leads to invasive Trichosporon infections to cause a positive result on cryptococcal antigen testing[10]. Despite this similarity, Trichosporon GXM is relatively understudied and there is limited understanding of its role in detecting infection or monitoring response to therapy[9]
Treatment of infection with Trichosporon asahii can be challenging due to significant antifungal resistance. One study found that 47% of included isolates of T. asahii had amphotericin B MIC of > 2 mcg/mL and most isolates also had high MIC for caspofungin and flucytosine[11]. The azoles are the family of drugs with the most activity against T asahii, with voriconazole considered the most effective[2,11,12]. However, there has been at least one case where a patient intolerant of voriconazole was successfully treated with isavuconazole[13]. Breakthrough infection with T. asahii in a patient receiving itraconazole prophylaxis has been reported[14]. Combination therapy with multiple classes of antifungal medication has also been reported, with one patient able to undergo reduced intensity bone marrow transplant without relapse of fungemia after treatment with liposomal amphotericin B and voriconazole[15].
Even with appropriate antifungal therapy, the mortality associated with Trichosporon infections has been reported in excess of 80%, with particularly poor outcomes in patients who remain neutropenic[7,12]. Limited evidence has suggested longer survival in those who receive therapy with azoles[16]. Optimal duration of treatment is unknown[5].
Key References:
1. Henter J-I, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer 2007; 48:124–131.
2. Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O. ESCMID† and ECMM‡ joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 2014; 20:76–98.
3. Li H, Guo M, Wang C, et al. Epidemiological study of Trichosporon asahii infections over the past 23 years. Epidemiol Infect 2020; 148:e169.
4. Girmenia C, Pagano L, Martino B, et al. Invasive Infections Caused by Trichosporon Species and Geotrichum capitatum in Patients with Hematological Malignancies: a Retrospective Multicenter Study from Italy and Review of the Literature. J Clin Microbiol 2005; 43:1818–1828.
5. Kim J, Kim MJ, Chong YP, et al. Comparison of the characteristics of patients with invasive infections and noninvasive infections caused by Trichosporon asahii. Med Mycol 2020; :myaa076.
6. Fleming RV, Walsh TJ, Anaissie EJ. Emerging and less common fungal pathogens. Infect Dis Clin North Am 2002; 16:915–933.
7. Walsh TJ, Groll A, Hiemenz J, Fleming R, Roilides E, Anaissie E. Infections due to emerging and uncommon medically important fungal pathogens. Clin Microbiol Infect 2004; 10:48–66.
8. Yamamoto M, Takakura S, Hotta G, et al. Clinical characteristics and risk factors of non-Candida fungaemia. BMC Infect Dis 2013; 13:247.
9. Fonseca FL, Frases S, Casadevall A, Fischman-Gompertz O, Nimrichter L, Rodrigues ML. Structural and functional properties of the Trichosporon asahii glucuronoxylomannan. Fungal Genet Biol FG B 2009; 46:496–505.
10. Melcher GP, Reed KD, Rinaldi MG, Lee JW, Pizzo PA, Walsh TJ. Demonstration of a cell wall antigen cross-reacting with cryptococcal polysaccharide in experimental disseminated trichosporonosis. J Clin Microbiol 1991; 29:192–196.
11. Chagas-Neto TC, Chaves GM, Melo ASA, Colombo AL. Bloodstream Infections Due to Trichosporon spp.: Species Distribution, Trichosporon asahii Genotypes Determined on the Basis of Ribosomal DNA Intergenic Spacer 1 Sequencing, and Antifungal Susceptibility Testing. J Clin Microbiol 2009; 47:1074–1081.
12. Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol 2004; 42:4419–4431.
13. Feugray G, Krzisch D, Dehais M, et al. Successful treatment of Trichosporon asahii fungemia with isavuconazole in a patient with hematologic malignancies. Infect Drug Resist 2019; 12:2015–2018.
14. Karigane D, Sakurai M, Matsuyama E, et al. Successful treatment of breakthrough disseminated Trichosporon asahii fungemia in a patient with acute myeloid leukemia receiving itraconazole prophylaxis. Med Mycol Case Rep 2018; 20:1–3.
15. Hosokawa K, Yamazaki H, Mochizuki K, et al. Successful treatment of Trichosporon fungemia in a patient with refractory acute myeloid leukemia using voriconazole combined with liposomal amphotericin B. Transpl Infect Dis Off J Transplant Soc 2012; 14:184–187.
16. Suzuki K, Nakase K, Kyo T, et al. Fatal Trichosporon fungemia in patients with hematologic malignancies. Eur J Haematol 2010; 84:441–447.
