Title: Protracted illness – A 70-year-old immunocompetent man with invasive Candidiasis
Submitted by: Jan Grothe, Liv Inga Hamann, Oliver Cornely
Institution: University Hospital Cologne
Email: jan.grothe@uk-koeln.de
Date Submitted: 06/09/2022
History:
A 70-year-old gentleman was admitted to a German Military Hospital in early January 2022 with multiple lacerations of the head and retrograde amnesia following a fall at his nursing home. During the last weeks the patient had become increasingly disoriented and imbalanced. CT scans of the head excluded cerebral hemorrhage and showed slight ventricular enlargement. He was thus transferred to the neurosurgical ward and underwent lumbar puncture in order to rule out normal pressure hydrocephalus. CSF analysis revealed an elevated tau protein of 925,47pg/ml (normal range <445pg/ml) and signs of a disrupted blood-brain barrier. Further testing including an EEG, TTE and MRI of the head remained largely unremarkable. At the same time an elevated CRP and slight increase of body temperature was noted. It was decided to collect blood cultures and initiate cefuroxime 500mg iv bid.
Past medical history (essentials):
09/2012 – Valve sparing aortic root surgery (Yacoub procedure)
01/2021 – Multiple cardiogenic bihemispheric cerebral emboli (fibrinolytic therapy)
08/2021 – Transcatheter aortic valve implantation
11/2021 – Left acetabular fracture, dislocation of the hip prosthesis
– ORIF surgery with a plate
12/2021 – ICU in a university hospital
– Urinary tract infection
– Acute Kidney Injury
– Atypical diabetic ketoacidosis
Physical Examination:
The general physical exam was unremarkable, despite for a low-grade fever and discomforting gluteal pain when sitting.
The neurological exam revealed disorientation, gait disturbance and paresis of left leg. Left Achilles and patellar reflex were absent.
Laboratory Examination:
Slightly elevated CRP.
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed:
Several blood cultures revealed Candida glabrata. Candida glabrata had also been detected in a blood culture one month earlier but was unfortunately overlooked or wrongfully assessed as a contaminant and left untreated by the critical care team of another nearby hospital.
Following the diagnosis of candidemia, evaluation for metastatic infection was initiated. TEE revealed a vegetation on the biological aortic valve. Brain MRI was repeated and displayed several acute and subacute infarctions in the frontal and occipital region as well as the basal ganglia, highly suggestive of septic emboli. At the same time the patient status deteriorated and required intensive care. Further diagnostics included a PET/CT in which only a minor nucleotide covering of the aortic valve was observed but also additional uptake in what appeared to be an encapsuled formation in the left gluteal region. The latter was presumably caused by a protruding screw from the recent ORIF surgery. Several punctures did not yield usable material and an abscess was excluded by the surgical consultants.
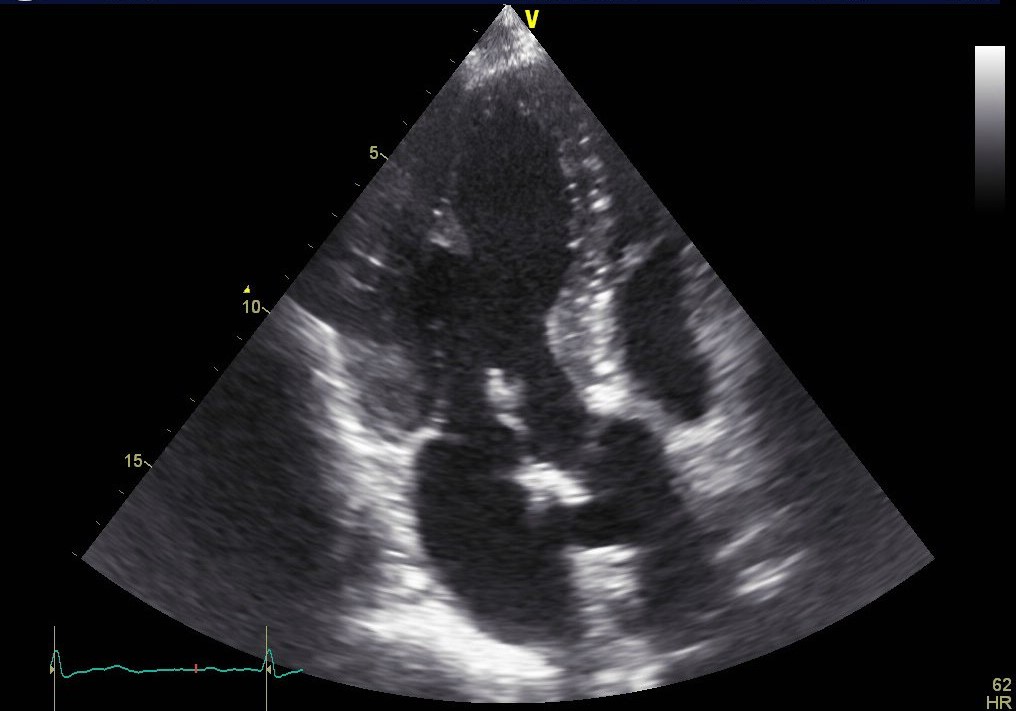
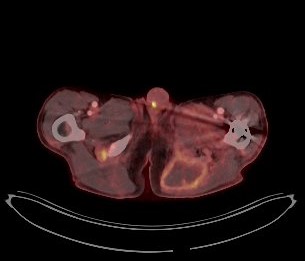
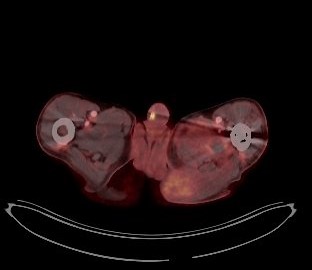
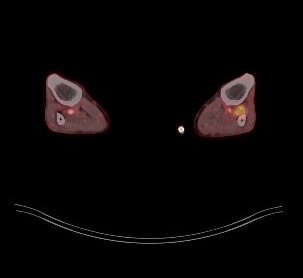
Once the patient had been stabilized and antifungal treatment started, the biological aortic valve was replaced with a mechanical valve. Histopathology and culture from a valve sample confirmed the destructive growth of fungal hyphae and Candida glabrata as the causative agent. Subsequent PET/CT scans revealed a persistent retrosternal fluid buildup as well as two additional smaller formations in the left thigh and lower leg, which may also explain a newly found paresis.
Final Diagnosis:
Acute Invasive Candidiasis in the form of fungemia, endocarditis, septic brain emboli and presumably multiple encapsulated muscular dissemination.
Question 2: What treatment is recommended in the care of this patient?
Treatment:
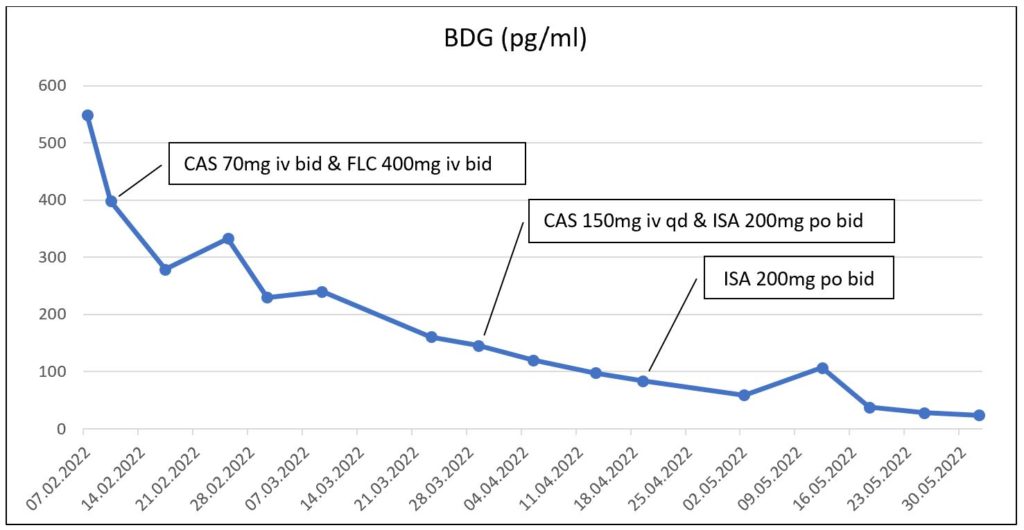
The initial antifungal therapy consisted of a combination of caspofungin 70mg iv qd and fluconazole 400mg iv bid, which led to no noticeable improvement after 3 weeks. At this point our institution was asked to get involved. We advised to measure ?-D-glucan and increase the caspofungin dose to 150mg qd. Based on susceptibility testing, fluconazole was switched to isavuconazole 200mg po bid. As iv therapy became more and more difficult caspofungin was discontinued and oral isavuconazole monotherapy maintained.
Table 1. MICs
(CAS=Caspofungin, FLC=Fluconazole, ISA=Isavuconazole)
Outcome:
Serum ?-D-glucan concentration declined continuously and eventually reached values below 50pg/ml. Shortening of the bone screw as well as a puncture and surgical drain of several suspected Candida seedings in the left leg are in the planning stage.
Discussion:
With a worldwide increase of non-albicans species in nosocomial blood stream infections, Candida glabrata is an important yeast to be reckoned with. Several characteristics such as the strong adherence to epithelial tissue, biofilm formation and intrinsic resistance to azoles make it a difficult to treat and even lethal pathogen.1
In this particular case we cannot tell with certainty what the route of infection was. However, the first positive blood culture was recorded one month prior to the diagnosis during a stay in an ICU. Several risk factors including a central venous catheter, antibiotic treatment, uncontrolled diabetes mellitus and acute kidney injury could have fostered this infection.2,3 An important learning lesson is that Candida in a blood culture should never be considered a contaminant.4 On the contrary, proper workup along with a search for the source of infection, echocardiography, eye examination and the removal of all indwelling lines is essential.5,6 Such intricate clinical management can be supported and improved by the EQUAL Candida Score, which is a clinical tool for antifungal stewardship and guideline adherence.7
The atypical clinical presentation with signs of chronic neurodegeneration made the diagnosis unusually tricky. Some research suggests that low-grade candidemia in mice can cause mild memory impairment.8 Candida albicans has been shown to cross the blood brain barrier and cause transient cerebritis and formation of parenchymal plaques composed of amyloid ? peptides.8,9 Indeed, once antifungal treatment was effectively applied, an astonishing improvement of cognitive functions was observed in this patient.
The initial choice of antifungal treatment and dosing was marked by inexperience and illustrates the need for infectious disease specialists to get involved in such critical cases. Although Candida glabrata is known to possess natural resistance to azoles, isavuconazole has been shown to have high activity in vitro.10,11 In view of only limited options for oral step-down therapy isavuconazole should be kept in mind and looked out for in susceptibility testing. It has not yet been approved for this indication but may be a promising candidate.
Given that Candida glabrata can develop azole resistance during therapy, we intended treatment with more than one antifungal agent.12 In this case we had flucytosine at our disposal. Susceptibility for flucytosine was adequate but dismissed because of the need for therapeutic drug monitoring in an outpatient setting. The patient recovered nonetheless clinically and with regard to his laboratory values.
Key References:
1. Rodrigues CF, Silva S, Henriques M. Candida glabrata: a review of its features and resistance. Eur J Clin Microbiol Infect Dis. 2014;33(5):673-688.
2. Poissy J, Damonti L, Bignon A, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care. 2020;24(1):109-109.
3. Thomas-Rüddel DO, Schlattmann P, Pletz M, Kurzai O, Bloos F. Risk Factors for Invasive Candida Infection in Critically Ill Patients: A Systematic Review and Meta-analysis. Chest. 2022;161(2):345-355.
4. Kauffman CA. Epidemiology and pathogenesis of candidemia in adults. In: Marr KA, ed. UpToDate, Waltham, MA.2022.
5. Cornely OA, Bassetti M, Calandra T, et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37.
6. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62(4):e1-50.
7. Mellinghoff SC, Hoenigl M, Koehler P, et al. EQUAL Candida Score: An ECMM score derived from current guidelines to measure QUAlity of Clinical Candidaemia Management. Mycoses. 2018;61(5):326-330.
8. Wu Y, Du S, Johnson JL, et al. Microglia and amyloid precursor protein coordinate control of transient Candida cerebritis with memory deficits. Nat Commun. 2019;10(1):58.
9. Parady B. Innate Immune and Fungal Model of Alzheimer’s Disease. J Alzheimers Dis Rep. 2018;2(1):139-152.
10. Marcos-Zambrano LJ, Gómez A, Sánchez-Carrillo C, et al. Isavuconazole is highly active in vitro against Candida species isolates but shows trailing effect. Clin Microbiol Infect. 2018;24(12):1343.e1341-1343.e1344.
11. Seifert H, Aurbach U, Stefanik D, Cornely O. In vitro activities of isavuconazole and other antifungal agents against Candida bloodstream isolates. Antimicrob Agents Chemother. 2007;51(5):1818-1821.
12. Barber AE, Weber M, Kaerger K, et al. Comparative Genomics of Serial Candida glabrata Isolates and the Rapid Acquisition of Echinocandin Resistance during Therapy. Antimicrob Agents Chemother. 2019;63(2):e01628-01618.
