Title: Fever of Unknown Etiology and Pancytopenia in a Solid Organ Transplant Recipient.
Submitted by: Amy Spallone, MD, Laila Woc-Colburn, MD
Institution: Baylor College of Medicine
Email: amy.spallone@bcm.edu
Date Submitted: April 2019
History: 34 y/o Hispanic male was admitted for worsening pancytopenia over the past two weeks. His past medical history was significant for alcoholic cirrhosis leading to orthotopic liver transplantation in August 2017. Two weeks prior to this presentation he was admitted for fevers without any associated complaints. He underwent an extensive infectious disease work up, which revealed he was Rhinovirus positive on respiratory viral panel. Patient was treated with supportive care at the time. On his current admission, patient states that he was been having persistent and daily fevers since his last hospitalization.
Medications: Prednisone 17.5mg daily and tacrolimus 1mg BID. His mycophenolic acid was stopped a few days prior to admission due to a downward trend of his white blood cell count.
Social History: He lives in Houston, TX with his family. He recently started mowing the lawn and landscaping. He denies animal exposures.
Physical Examination:
BP: 136/69 mmHg
Pulse: 138/min
Resp: 20/min
Temp: 102.3 °F (39.1 °C)
SpO2: 97% on 5L supplemental oxygen
GENERAL: Appears ill and diaphoretic, shivering
HEENT: PERRL, anicteric, oral mucosa moist w/o ulcers/thrush. Poor dentition.
NECK: Full ROM, no rigidity or pain on flexion CVS: Tachycardic, S1S2 present, no murmurs, rubs or gallops. No pedal edema.
RESP: clear to auscultation and percussion. GI: Abdomen was soft and non-tender; no organomegaly.
SKIN: No rash or ulceration, with abundant tattoos. Extremities: no gross deformity, no limitation of ROM.
Laboratory Examination:
CBC:
WBC 0.8
RBC 2.73
HGB 7.5
HCT 23.4
MCV 85.7
CMP:
NA 133
K 4.9
CL 102
CO2 22
BUN 51
PHOS 5.3
AST 18
ALT 32
Radiology: CT Chest without IV contrast: The mediastinum demonstrates multiple small lymph nodes but no suspicious masses or adenopathy. The pulmonary parenchyma demonstrates diffuse reticulonodular markings throughout the lung fields which are new from previous. Multifocal ground glass and airspace opacities are seen in the left upper lobe with areas of mosaic attenuation.
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed:
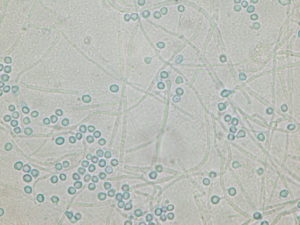
Bronchoscopy with lavage: NUMEROUS PREDOMINANTLY INTRACELLULAR YEAST FORMS, SUGGESTIVE OF HISTOPLASMA SPECIES
Histoplasma Antigen: >25.0 ng/mL
Final Diagnosis: Disseminated histoplasmosis in a solid organ transplant patient
Question 2: What treatment is recommended in the care of this patient? Treatment: Liposomal amphotericin B 3 mg/kg/day, methylprednisolone 1.0 mg/kg/day adjunctive therapy
Outcome: Fevers and leukopenia resolved on liposomal amphotericin B. Hospital course was complicated by acute respiratory failure due to ARDS requiring mechanical ventilation, hemodynamic shock requiring ECMO, and acute kidney injury. Patient slowly improved with therapy and supportive care.
Discussion: (500 words)
Histoplasma capsulatum, the cause of histoplasmosis, is a dimorphic fungus with worldwide distribution. It is one of the most common systemic endemic mycosis in the United States. It is found in the central regions of the United States, from the Gulf Coast to the Great Lakes. Infection is caused by the inhalation of environmental microconidia which are frequently found in the soil containing decayed bird or bat guano. After inhalation, it is taken up by the alveolar macrophages where they can disseminate through the lymphatics and bloodstream. Infections can range widely from asymptomatic exposure to life-threatening disease depending on a number of factors, such as inoculum and integrity of the host’s immune system. Healthy subjects develop cellular immunity after exposure and recover without significant morbidity. In solid-organ transplant (SOT) patients, the burden of disease is often out of proportion to the severity of symptoms at presentation (1,2,5).
Although Histoplasmosis is common, it occurs in <1% of transplant recipients, even in endemic areas. The majority of cases of Histoplasmosis in SOT patients represent an exogenous infection or a reactivation. However, donor derived infection has also been described. T cell immunity represents the primary host defense against Histoplasma spp. and is generally reduced in SOT patients who are on T cell suppressant therapy (e.g. such as tacrolimus in our patient). This inability to mount an effective cellular response may lead to the patient presenting with overwhelming disseminated infection characterized by extra-pulmonary dissemination (2,3,5).
Respiratory manifestations are typically present and radiography demonstrates a diffuse reticulonodular or miliary pattern. Other common manifestations include fever, weight loss, hepatosplenomegaly, lymphadenopathy, and cutaneous and mucosal lesions. Laboratory manifestations of infection includes bone marrow suppression and hepatic inflammation. The typical period from onset of symptoms to diagnosis is frequently 2-4 weeks. Additionally, infection tends to occur within the first two years following SOT. Severe Histoplasmosis infections can present as shock, respiratory failure, and consumptive coagulopathy and are frequently misdiagnosed as septic shock due to a bacterial infection.
Definitive diagnosis is made with the direct visualization of yeast forms or the growth of Histospama spp. from specimens. Isolating the organism from tissue or body fluids, such as bronchoalveolar lavage, as in our patient, can expedite the diagnosis. Newer antigen assays have also improved the early detection of infection with increased sensitivity and specificity. The urinary Histoplasma antigen EIA appears to have the highest overall sensitivity at 92%. However, the sensitivity is lower in patients with isolated pulmonary disease when compared to disseminated infection (2,4, 5).
Liposomal amphotericin is recommended for moderate to severe infections and should be continued for 1-2 weeks or until the patient stabilizes. Afterwards, oral step down therapy with itraconazole (200mg twice daily) for a minimum of 1 year is recommended. It is also imperative, that every effort should be made to reduce the degree of immunosuppression (especially calcineurin inhibitors) during acute infection period (2,3,5). Paradoxical worsening of symptoms after the initiation of antifungals is consistent with immune reconstitution inflammatory syndrome and may occasionally be seen.
Key References:
- Benedict, K, Rajal, K, Mody, RK. Epidemiology of Histoplasmosis Outbreaks, United States, 1938–2013. Emerg Infect Dis, 22(3), 370-378.
- Hage, CA, Pescovitz, L, & Wheat, J. Histoplasmosis in Solid Organ Transplant Patients. Retrieved April 5, 2019, <http://www.antimicrobe.org/new/t17_dw.html>
- Assi M, Martin S, Wheat LJ, et. al. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013 Dec;57(11):1542-9.
- Simon, CT, et. al. Unexpected disseminated histoplasmosis detected by bone marrow biopsy in a solid organ transplant patient. Clin Case Rep. 2018 Jan; 6(1): 49–51.
- Miller, R, & Assi, M (2019). Endemic Fungal Infections in Solid Organ Transplant Recipients – Guidelines from the American Sociaety of Transplantation Infectious Diseases Community of Practice. Clinical Transplantation. doi:10.1111/ctr.13553
