Yeast in the urine: don’t be tricked by a rare pathogen
Submitted by: Jack McHugh MB BCh BAO, Paschalis Vergidis MD
Institution: Mayo Clinic, Rochester, Minnesota
Email: mchugh.jack@mayo.edu
History:
A 68 year old male was admitted for ultrasonic lithotripsy. He had developed chronic renal stones in the setting of suprapubic catheter placement for neurogenic bladder. Subsequent recurrent urinary tract infections were managed with trimethoprim-sulfamethoxazole suppressive therapy. Medical history was notable for multiple sclerosis, and diabetes mellitus managed with oral hypoglycemic agents.
Approximately 8 hours following the procedure, the patient developed tachycardia, hypoxia, and a low-grade fever. He was initiated on vancomycin, gentamicin, and levofloxacin for empiric treatment of suspected sepsis. He was transferred to the ICU where he received fluid resuscitation and blood pressure support with vasopressors.
Physical Examination:
HR: 119, RR: 18 BP: 92/52mmHg, SpO2 93% on 50% O2 via face mask, T 38.1C
General: awake, drowsy, confused
Heart: tachycardia, regular rhythm, no rubs, murmurs, or gallops appreciated
Lungs: minimal breath sounds heard over right lung, clear on left
Abdomen: soft, non-tender, non-distended, positive bowel sounds
Genitourinary: suprapubic and percutaneous nephrostomy catheter sites clean, no discharge or peri-catheter erythema
Remainder of the physical examination was unremarkable
Laboratory Examination:
Initial laboratory results are presented in Table 1. Cultures were taken from blood and suprapubic catheter. ECG showed sinus rhythm. Chest x-ray was notable for small bilateral pleural effusions, bibasilar atelectasis, with no definite focal infiltrate.
| Table 1. Laboratory data | ||
| Variable | Reference Range(Male Adults) | At Time of Deterioration |
| Hemoglobin (g/dl) | 12.3-16.6 | 8.3 |
| White Cell Count (x109/L) | 3.4-9.6 | 9.2 |
| Platelet Count (x109/L) | 135-317 | 256 |
| Sodium (mmol/L) | 135-145 | 138 |
| Potassium (mmol/L) | 3.6-5.2 | 4.0 |
| Chloride (mmol/L) | 98-107 | 108 |
| Bicarbonate (mmol/L) | 22-29 | 26 |
| Urea Nitrogen (mg/dl) | 8-24 | 15 |
| Creatinine (mg/dl) | 0.74-1.35 | 1.0 |
| Glucose (mg/dl) | 70-140 | 159 |
Case Continuation: On day 2 of ICU admission blood cultures demonstrated growth of yeast. Caspofungin was started, and vancomycin and gentamicin were discontinued. The patient continued to experience low grade fevers to 37.8°C, and required oxygen via nasal cannula. Repeat chest X-ray the following day showed bilateral perihilar and bibasilar infiltrates, and bronchoalveolar lavage (BAL) was performed. Cultures from the suprapubic catheter also demonstrated growth of yeast. Anti-fungal treatment was switched to fluconazole 400mg once daily. A diagnostic result was obtained.
Question 1: What are probable/possible diagnoses?
This patient exhibits signs of sepsis associated with fungemia and funguria; the likely source is colonization of his suprapubic catheter. Preliminary cultures show growth of yeast, and he has failed treatment with an echinocandin. While the infiltrates on chest x-ray may be related, bacterial co-infection is a strong possibility. The differential diagnosis for a disseminated yeast infection is relatively narrow, and the following pathogens should be considered.
Candida spp. is the most common cause of fungemia in the USA, and the fourth most common cause of nosocomial bloodstream infection in the USA1. Candiduria is common in hospitalized patients and candidemia may occur following procedures involving the urinary tract. While not first line for treating Candida urinary tract infections, echinocandins have shown efficacy similar to that of fluconazole2. Diabetes, present in this patient, is an important risk factor for invasive Candida infection.
Cryptococcus spp. is a common cause of yeast infection in the immunosuppressed population, particularly in those with HIV infection3. While it may affect any organ, by far the most likely presentation is meningoencephalitis. C. neoformans may very rarely infect the urinary tract and should be considered in the differential.
Trichosporon spp. is an emerging pathogen that may present with disseminated disease, or most commonly with pulmonary lesions when a single organ is affected4. Of note, echinocandins are universally ineffective in the treatment of this yeast5.
Additional yeasts that may cause fungemia include Rhodotorula spp., Malassezia spp., and Lachancea spp.
Microbiology/Diagnostic Tests Performed:
- Blood Cultures:
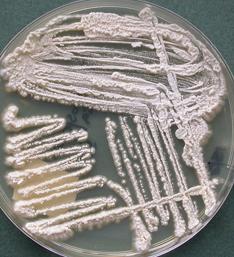
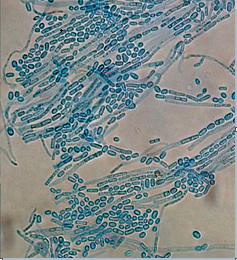
- Positive in 1 of 2 bottles: Trichosporon Asahii (Figures 1a,1b)
- Sensitivities:
- S= Susceptible; I = Intermediate; R = Resistant; D = Susceptible dose dependent; N= Not susceptible. Susceptibilities in ?g/ml
- Caspofungin >8 N, Flucytosine 1 S, Posaconazole 0.25 S, Voriconazole 0.06S, Itraconazole 0.25 D, Fluconazole 4 S, Amphotericin-B 0.5 S
- Urine, Catheterized Specimen: T. asahii, many
- Suprapubic Catheter Skin site: T. asahii, many
- BAL: no growth noted
Final Diagnosis:
Trichosporon asahii fungemia of urinary tract source
Question 2: What treatment is recommended in the care of this patient?
Treatment:
Treatment of trichosporonosis is challenging in the immunosuppressed host. The optimal agent and duration of treatment have not been fully elucidated. Voriconazole has been suggested as a first line agent given its increased activity over other azoles in-vitro6,7, in addition to clinical data suggesting favorable outcomes4. Amphotericin B is an alternative second line agent, although varying susceptibilities have been reported8. In severe infections aggressive treatment is warranted. A suggested regimen is the combination of voriconazole and amphotericin B for two weeks followed by transition to oral voriconazole with duration of therapy determined by clinical response.
As mentioned previously, the echinocandins have virtually no efficacy against this organism. Multi-drug resistant strains have been successfully treated with combination liposomal amphotericin B and flucytosine9. The patient had responded well to fluconazole prior to consultation, and a decision was made to continue this course of treatment.
Outcome:
The patient continued on fluconazole for a two week course, and levofloxacin for a one week course. Lung infiltrates improved on subsequent chest x-ray, and hypoxia improved. BAL did not show growth of bacteria or fungi. He was discharged in a stable condition on day 13 of his hospital course.
Discussion:
Gentlemen of high status in 19th century France became accustomed to removing small white nodules from the base of their wigs. These nodules came to be known as white piedra (Spanish – stone), and the causative agent was subsequently identified in 1865 as Trichosporon spp.10. With the increased prevalence of immunosuppression, this genus of basidiomycete yeast-like fungi has emerged as an important cause of disseminated fungemia, with estimated mortality rates of 50-80%.
Trichosporon is comprised of 34 species, of which 6 cause clinically relevant disease in humans. Most commonly however Trichosporon spp. form part of the normal flora of mouth, skin and nails. Rates of colonization in patients admitted to general wards vary from 1-3%11,12. Disease may be classified as superficial or invasive. T. asteroids and T. cutaneum are associated with superficial skin infections. T. inkin and T. ovoides are associated with white piedra. T. asahii is the most common cause of invasive disease, accounting for approximately three quarters of Trichosporon infection cultured from urine or blood4. Invasive disease may affect any organ, with lung involvement most commonly seen. Immunosuppression is common in this cohort and underlying hematologic disease has been reported as the most frequent risk factor4. In this case the host had a background of diabetes and was on chronic antibiotics. Most notably however he was undergoing a urologic procedure for management of nephrolithiasis, and had a long-term suprapubic catheter. While rare, urinary tract infections with T. asahii have been reported in the immunocompetent hostin the presence of a long-term catheter13. In this case positive skin cultures obtained from the catheter site suggest colonization and subsequent biofilm formation.
Although most infections occur in the immunosuppressed host, our patient did not have a significantly immunocompromising condition. The ability of Trichosporon spp. to form biofilms and colonize indwelling devices has been reported frequently14,15, and this pathogen should be considered in cases of fungal growth from an indwelling device. This case also demonstrates the failure of echinocandins in the treatment of Trichosporon spp. These agents are commonly used for the empiric treatment of yeast; here we highlight the limitations of this treatment modality.
Key References:
- Wisplinghoff H, Bischoff T, Sandra T, et. al., Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin Infect Dis. 2004; 39(3):309–317
- Cuervo G, Garcia-Vidal C, Puig-Asensio M, et. Al. Echinocandins Compared to Fluconazole for Candidemia of a Urinary Tract Source: A Propensity Score Analysis, Clin. Inf. Diseases. 2017; 64(10):1374–1379
- Abadi, J, Nachman AB, Kressel, Pirofski L. Cryptococcosis in children with AIDS. Clin Infect Dis. 1999; 28:309-313.
- de Almeida Júnior JN, Hennequin C. Invasive Trichosporon Infection: a Systematic Review on a Re-emerging Fungal Pathogen. Front Microbiol. 2016. 7:1629.
- Matsue K, Uryu H, Koseki M, Asada N, Takeuchi M. Breakthrough trichosporonosis in patients with hematologic malignancies receiving micafungin. Clin Infect Dis. 2006; 42:753-757
- Hazirolan G, Canton E, Sahin S, Arikan-Akdagli S. Head-to-head comparison of inhibitory and fungicidal activities of fluconazole, itraconazole, voriconazole, posaconazole, and isavuconazole against clinical isolates of Trichosporon asahii. Antimicrob Agents Chemother. 2013;57(10):4841–4847.
- Francisco EC, de Almeida Junior JN, de Queiroz Telles VR et al. Species distribution and antifungal susceptibility of 358 Trichosporon clinical isolates collected in 24 medical centres. Clin Microbiol Infect. 2019. 909.e1-909.e5
- Lemes RM, Lyon JP, Moreira LM, de Resende MA. Antifungal susceptibility profile of Trichosporon isolates: correlation between CLSI and etest methodologies. Braz J Microbiol. 2010;41(2):310–315.
- Walsh TJ, Newman KR, Moody M, Wharton RC, Wade JC. Trichosporonosis in patients with neoplastic disease. Medicine. 1986; 65(2);68-79
- Beigel H. 1865. Cited by Langeron M. In: Darier J, editor. Nouvelle Practique Dermatologique. Paris: Masson and Cie. 1936; 377
- Rose HD, Kurup VP. Colonization of hospitalized patients with yeast-like organisms. Sabouraudia. 1977;15(3):251.
- Haupt HM, Merz WG, Beschorner WE, Vaughan WP, Saral R. Colonization and infection with Trichosporon species in the immunosuppressed host. J Infect Dis. 1983;147(2):199.
- Khan ID, Sahni AK, Basu A, Haleem S. Trichosporon asahii urinary tract infection in immunocompetent patients. Med J Armed Forces India. 2015;71(4):373–376.
- Bongomin F, Out A, Calisti G, et al. Trichosporon japonicum Fungemia and Ventricular Assist Device Infection in an Immunocompetent Patient, Open Forum Infect Dis. 2019:6(9); ofz343
- Sun W, Su J, Xu S, Yan D. Trichosporon asahii causing nosocomial urinary tract infections in intensive care unit patients: genotypes, virulence factors and antifungal susceptibility testing. J Med Microbiol. 2012;61(12):1750-1757
