Title: Invasive Mucormycosis and the Dilemma of Combination Therapy: A Triple Threat Case Report – Influenza A Pneumonia, Bacterial Co-infection, and Invasive Mold disease
Figures: 2
Submitted by: Adrian Davies DO1, Divisha Sharma MD2 and Jose Vazquez MD2
Institution:
1Medical College of Georgia at Augusta University, Internal Medicine Residency Program?
2 Medical College of Georgia at Augusta University, Division of Infectious Diseases??
Email: adavies@augusta.edu
Date Submitted: 05/01/2023
Mucormycosis is a rare, life-threatening opportunistic infection predominantly affecting the immunocompromised population. In this case, we report a 68-year-old female with no discernible immunocompromising condition, apart from uncontrolled diabetes. She tested positive for influenza A viral infection, accompanied by methicillin-sensitive Staphylococcus aureus (MSSA) pneumonia, and subsequently unfolding pulmonary mucormycosis without rhino-cerebral involvement.
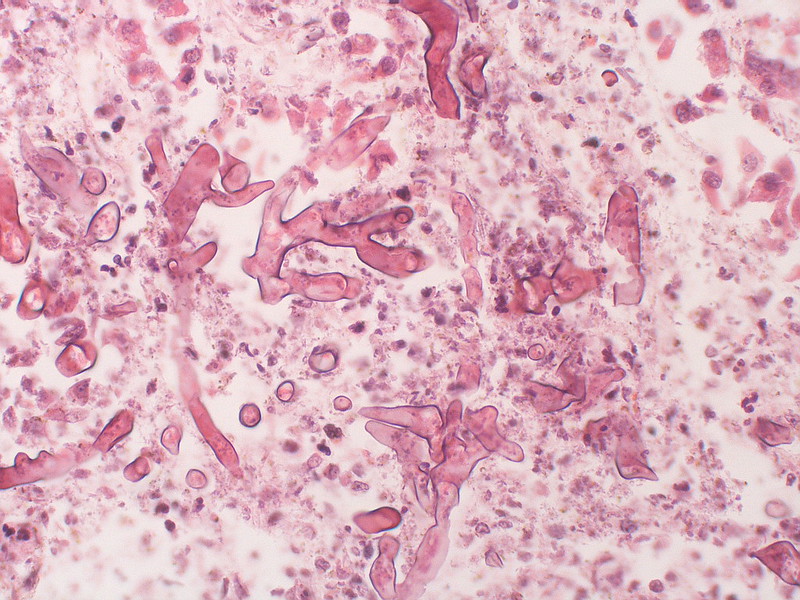
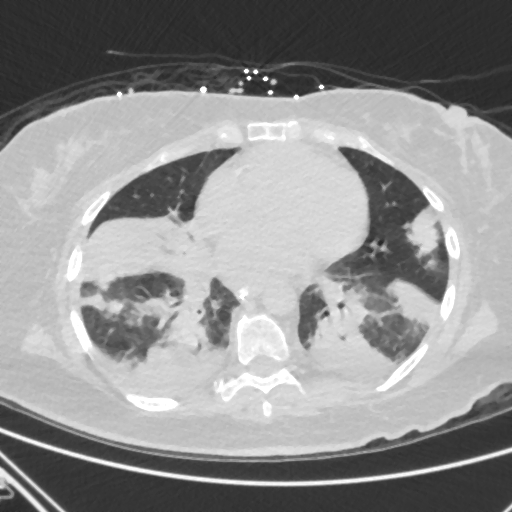
A 68-year-old female with a history of coronary artery disease and type II diabetes mellitus presented to the hospital with severe hyperglycemia and acute hypoxic respiratory failure. Initial laboratory findings were remarkable for a blood glucose of 1,074 with metabolic acidosis and a hemoglobin A1c of 14.8%. Her COVID-19 rapid test was negative; however, she tested positive for influenza A virus infection. Computed tomography (CT) of the chest demonstrated bilateral consolidations and diffuse ground-glass opacities, consistent with pneumonia [figure 1]. She was initially managed with empiric broad-spectrum intravenous (IV) antibiotics and the antiviral agent oseltamivir. Despite treatment, she remained febrile, hypoxic, and decompensated, necessitating endotracheal intubation. Bronchoalveolar lavage culture revealed methicillin-sensitive Staphylococcus aureus (MSSA) and mold growth, later identified as Mucor species. The infectious diseases team recommended tissue biopsy and initiation of IV isavuconazole in the meantime. Bronchoscopy revealed a right bronchial tree lesion and a gray-white film covering the right middle and bilateral lower lobes. Biopsy of the lesions demonstrated invasive necro-inflammatory disease with numerous visible fungal elements [figure 2]. CT scans of the head and maxillofacial region were obtained to rule out rhino-cerebral involvement. IV liposomal amphotericin B (LAmB) was initiated for invasive pulmonary mucormycosis while continuing isavuconazole, along with IV antibiotics for MSSA treatment. Surgical resection for source control could not be achieved as the patient had extensive bilateral lung involvement. She was eventually transferred to Mayo Clinic per the family’s request but unfortunately expired shortly thereafter.
Question 1: What are probable/possible diagnoses?
Microbiology/Diagnostic Tests Performed: Lung biopsy
Final Diagnosis: Invasive Pulmonary Mucormycosis
Question 2: What treatment is recommended in the care of this patient?
Treatment: IV Liposomal amphotericin B and Isavuconazole
Outcome: Fatal
Discussion:
Mucormycosis is a rare but aggressive fungal infection that predominantly affects patients with hematological malignancy, hematopoietic stem cell transplants, and other severely immune-compromising states. Over half of the patients who develop invasive mucormycosis typically present with rhino-cerebral manifestations initially. Pulmonary, cutaneous, gastrointestinal, and disseminated diseases are less commonly involved. Reports of invasive fungal infections have been increasing in frequency since early 2021 due to emerging associations with COVID-19 viral pneumonia and its treatments. This patient’s history of poorly controlled diabetes and recent influenza A viral pneumonia likely contributed to the development of mucormycosis, as both conditions can predispose patients to secondary bacterial and fungal infections (1, 2).
The diagnosis of invasive mucormycosis is challenging due to its nonspecific clinical presentation and overlapping symptoms with other infections. Histopathologic examination is the gold standard for diagnosis. Early recognition and prompt initiation of antifungal therapy are crucial, as mucormycosis has a rapid clinical progression. Surgical debridement of the involved tissue should be performed whenever possible, but it can be difficult to obtain in critically ill patients with extensive tissue involvement. Addressing and managing underlying risk factors, such as diabetes and immunosuppression, is essential to minimize the risk of opportunistic infections in patients with complex clinical presentations.
In the last two decades, LAmB remains the cornerstone of primary therapy for invasive mucormycosis, but the mortality rates remain unacceptably high. The adverse drug reactions related to the administration of moderate to high-dose LAmB are also a clinical concern. This has stimulated interest in studying novel combinations of antifungal agents to determine whether better outcomes can be achieved. In recent years, combination antifungal strategies have shown favorable results in pre-clinical models. So far, we know that adjunctive therapy with echinocandins or isavuconazole to LAmB is promising, given the markedly improved survival in relevant animal models of mucormycosis and available retrospective or observational clinical data that are concordant with the preclinical models for both efficacy and safety. These offer hope to improve survival in patients with invasive disease but are not enough to serve as the basis for clinicians to develop them as the standard of care. Definitive, randomized controlled clinical trials should be encouraged to determine whether superior outcomes might be achieved with combination therapy (3-7).
In conclusion, invasive Mucorales infection remains a global public health threat, as even after prompt diagnosis, initiation of LAmB, and surgical intervention, mortality rates remain above 50%. The lack of definitive large-scale clinical trials comparing efficacy of combination therapy versus monotherapy places clinicians in a dilemma, even when preclinical trials and murine models support the use of combination strategies.
Key References:
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases.?Clin Infect Dis. 2005;41(5):634-653. doi:10.1086/432579
- Muthu V, Agarwal R, Dhooria S, et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis.?Clin Microbiol Infect. 2021;27(4):538-549. doi:10.1016/j.cmi.2020.12.035
- Gebremariam T, Gu Y, Singh S, Kitt TM, Ibrahim AS. Combination treatment of liposomal amphotericin B and isavuconazole is synergistic in treating experimental mucormycosis.?J Antimicrob Chemother. 2021;76(10):2636-2639. doi:10.1093/jac/dkab233
- Katragkou A, McCarthy M, Meletiadis J, Petraitis V, Moradi PW, Strauss GE, Fouant MM, Kovanda LL, Petraitiene R, Roilides E, Walsh TJ. In vitro combination of isavuconazole with micafungin or amphotericin B deoxycholate against medically important molds. Antimicrob Agents Chemother. 2014 Nov;58(11):6934-7. doi: 10.1128/AAC.03261-14. Epub 2014 Aug 18. PMID: 25136021; PMCID: PMC4249375.
- Gebremariam, T., Wiederhold, N. P., Alqarihi, A., Uppuluri, P., Azie, N., Edwards, J. E., & Ibrahim, A. S. (2017). Monotherapy or combination therapy of isavuconazole and micafungin for treating murine mucormycosis. Journal of Antimicrobial Chemotherapy, 72(2), 462-466. https://doi.org/10.1093/jac/dkw433
- Brad Spellberg et al. Combination therapy for Mucormycosis. Clinical Infectious Diseases, 2012 Feb;54 Suppl 1 :S73-8.doi: 10.1093/cid/cir885.
- Durga Shankar Meena et al. Combination therapy in Mucormycosis. Journal of Medical Mycology, 2023 March Vol 33, issue 1. https://doi.org/10.1016/j.mycmed.2022.101332.
