| MSGERC Case of the Month |
|
Title: A Bold Mold Takes Hold Submitted by: Emily M. Eichenberger, Jennifer L. Saullo, John R. Perfect, Danielle Brander, Julia M. Messina Institution: Duke University Medical Center Email: Emily.Eichenberger@duke.edu Date Submitted: 9/30/2019 |
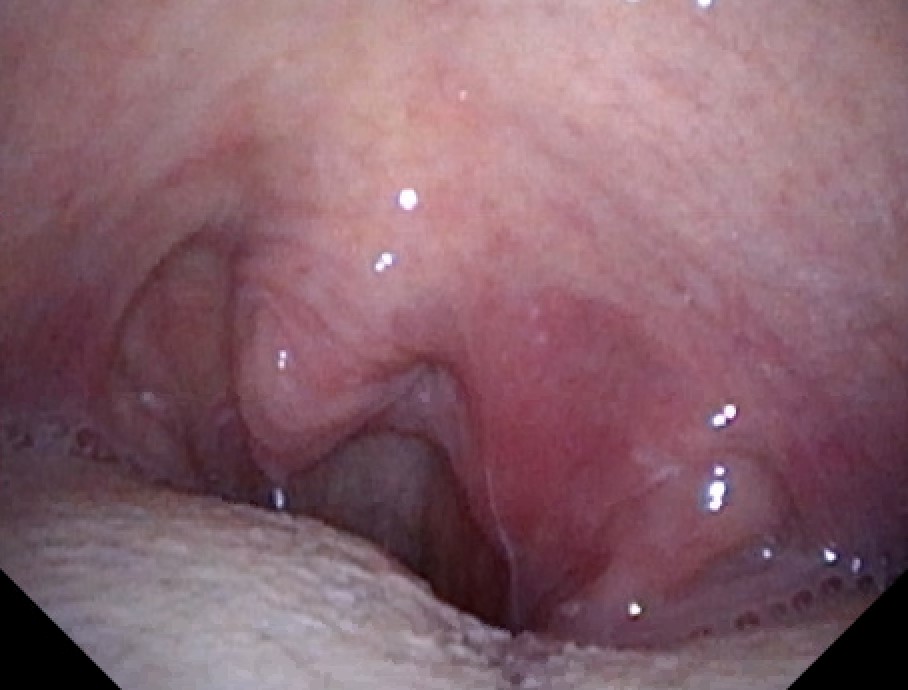
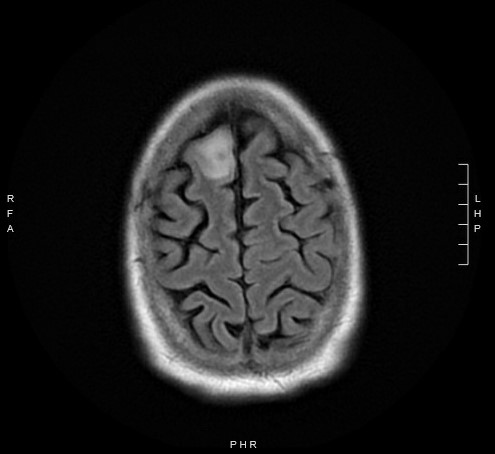
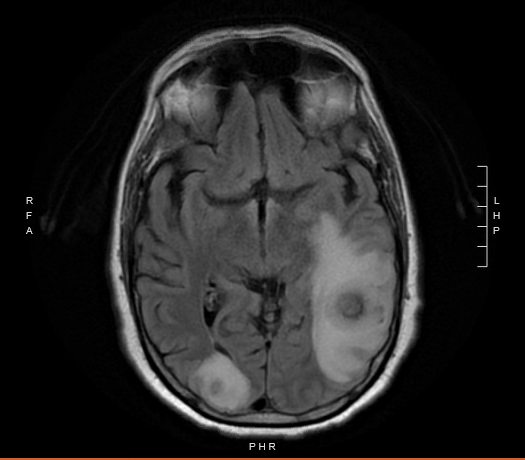
| History: 62-year-old man with newly diagnosed chronic lymphocytic leukemia (CLL) on Ibrutinib therapy for 1 month prior to presentation with fevers, aphasia and a new brain lesion. He was doing well on the therapy for approximately 3 weeks until he developed a sore throat, and neck swelling. He was seen by an otolaryngologist who described a left peritonsillar abscess [Figure 1]. An aspiration of the left peritonsillar abscess was attempted to alleviate the swelling, but no purulence was aspirated. He was instructed to take amoxicillin-clavulanate for 10 days. While nearing the end of the antibiotic therapy, the patient began to have intermittent fevers and progressive word finding difficulty. His symptoms progressed to confusion and profound expressive aphasia. He was subsequently admitted to the hospital for work up. A non-contrast head CT was obtained which demonstrated a large 3.2 centimeter (cm) hyperdense mass in the left cerebral hemisphere with surrounding vasogenic edema, a mass effect on the left lateral ventricle, as well as small hyperdense masses in the right cerebral hemisphere. The patient was started on dexamethasone and levetiracetam. An MRI of the brain with and without contrast demonstrated 3 enhancing mass lesions with surrounding vasogenic edema. Specifically, there was a 3.2 cm round heterogeneous mass noted in the left parietotemporal region, a 12 mm (millimeter) mass in the right occipital lobe, and an oval shaped 8mm mass in the right frontal lobe. [Figure 2A and 2B]. Infectious diseases was consulted for diagnostic and therapeutic recommendations. Physical Examination: General: confused and not fully cooperative with exam Psych: unable to state name, location or time Eyes: 5 mm reactive pupil OD, 5 mm non-reactive pupil OS (baseline), estropia OS with limited abduction (baseline), conjunctiva clear, anicteric sclera Neck: normal, supple, no adenopathy and mild tenderness to palpation over the left anterior cervical region HENT: Slight deviation of the uvula to the right, posterior ecchymoses in the oropharynx, pinpoint area of white plaque on the posterior pharynx Cardiovascular: regular rate and rhythm, without murmurs, rubs or gallops Respiratory: clear to auscultation bilaterally; good air exchange Abdomen: soft, nontender, nondistended, normoactive bowel sounds Extremities: no lower extremity edema Skin: no rashes Neurologic: cranial nerves intact (save baseline abnormalities OS as documented above), strength symmetric (5/5 throughout) with unsteady gait. Mild dysarthria. Difficulties with word finding, repetition and following commands. Great difficulty reading words but recognized pictures, unable to recognize a coin placed in his right hand. Laboratory Examination: WBC 3.2×10^9/L [ref range 3.2-9.8×10^9/L: Differential: 60% neutrophils [ref range 37-80%] 1% bands [ref range 0-6%] 1% basophils [ref range 0-2%] 1% eosinophils [ref range 0-7%] 34% lymphocytes [ref range 10-50%] 3% monocytes [ref range 0-12%] Hgb 11.6g/dL (ref range 13.7-17.3g/dL) MCV 229 fL [ref range 80-98 fL] Hct 32.3% [ref range 39.0-49.0-%] Plt 175 x10^9/L [ref range 150-450×10^9/L] Glucose 105 mg/dL [ref range 70-140 mg/dL] AST 20 U/L [ref range 15-41 U/L] ALT 16 U/L [ref range 17-63 U/L] Bilirubin, Total 0.6 mg/dL [ref range 0.4-1.5mg/dL] AlkPhos 51 U/L [ref range 24-110 U/L] |
| Question 1: What are probable/possible diagnoses? Differential diagnosis included: Central nervous system (CNS) cryptococcomas • Intracranial pyogenic abscesses • CNS toxoplasmosis •Neurocysticercosis • CNS aspergillosis • CNS tuberculomas • CNS nocardiosis • Metastatic CNS tumors • Primary CNS lymphoma Microbiology/Diagnostic Tests Performed: • Toxoplasma IgG non-reactive; IgM non-reactive • Toxoplasma PCR (whole blood): negative • Serum cryptococcal antigen: negative • Blood cultures: no growth • HIV antibody/antigen: negative • Serum Aspergillus galactomannan: <0.500 (negative) • Serum Beta-D Glucan Assay: <31 (negative) • CT chest (non-contrast) demonstrated a new spiculated nodule within the right lower lobe measuring 1.5 x 1.7 cm with mild surrounding glass opacity. • Brain biopsy histopathology demonstrated necro-inflammatory debris, hemorrhage and reactive gliosis, consistent with abscess. A GMS stain highlighted fungal hyphae. • Brain biopsy fungal culture with growth of Aspergillus fumigatus. Results of antifungal susceptibility testing were as follows: amphotericin B 0.5ug/ml, micafungin ?0.015ug/ml, posaconazole 0.5ug/ml, voriconazole 0.5ug/ml, isavuconazole 1ug/ml. Final Diagnosis: CNS Aspergillosis Question 2: What treatment is recommended in the care of this patient? Treatment: He was treated with over 1 year of voriconazole therapy with brief combination echinocandin (micafungin) therapy upfront for 2 weeks [1]. Ibrutinib was discontinued upon presentation and was held for the entire duration of Aspergillus treatment. Outcome: Serial CT and MRI imaging of the chest and brain respectively demonstrated excellent response to therapy with imaging at the close of 1 year of therapy with no suggestion of residual infection. Fortunately, the patients underlying CLL remained in good control during this period and he did not necessitate additional therapy with the exception of infrequent doses of granulocyte colony stimulating factor for intermittent mild neutropenia. He experienced multiple toxicities on voriconazole therapy including gastrointestinal disturbances, significant photosensitivity and nail changes. The patient also had a history of non-melanoma skin cancers; hence, the decision was made to transition to isavuconazole for secondary fungal prophylaxis after completion of over 1 year of voriconazole therapy. The transition to isavuconazole also occurred alongside impending plans by the oncology team to initiate venetoclax as his next line of CLL therapy Discussion: Here we present a case of CNS aspergillosis in a 62-year-old man with CLL who had initiated Ibrutinib 1 month prior to the diagnosis. Since the introduction of ibrutinib to treat CLL, multiple reports of invasive fungal infections have emerged [2-4]. Invasive aspergillosis typically starts with inhalation of Aspergillus conidia, followed by hyphal invasion of tissues and subsequent dissemination to other organs. With an intact innate immune system, alveolar macrophages recognize the Aspergillus cell wall components and phagocytose and kill conidia via oxidative mechanisms. The alveolar macrophages then secrete cytokines and chemokines such as TNF- and CXCR2 ligands, which recruit neutrophils to the site of infection. These neutrophils are integral to clearing the invasive fungal disease as hyphae are too large to be phagocytosed by the macrophages [5]. Ibrutinib is an irreversible Bruton’s tyrosine kinase (Btk) inhibitor that has demonstrated marked clinical efficacy in treating a variety of lymphoid malignancies. Btk plays a key role in B cell development, activation and proliferation. While the effect of ibrutinib on B cell function is widely understood and indeed harnessed for its anti-tumor properties, its role on macrophage and neutrophil function has drawn attention in the face of several reports of invasive fungal infections in patients on Ibrutinib. Aspergillus activates Btk in macrophages which in turn leads to downstream macrophage calcineurin-NFAT signaling to recruit neutrophils to the site of the Aspergillus infection [6]. However, when Btk is inhibited such as in the setting of Ibrutinib, the downstream NFAT and NF response is impaired, resulting in the lack of neutrophil recruitment [7]. Additionally, Blez, et al. have found that neutrophils harvested from patients treated with ibrutinib have significantly reduced neutrophil oxidative burst and absent IL-8 secretion in the setting of Aspergillus stimulation [8]. With an ibrutinib-impaired impaired innate immune system, the host cannot contain or clear Aspergillus infection, and the hyphae may spread and invade other organs. Given the finding of the new spiculated lung nodule, we suspect that our patient’s infection originated from inhalation of Aspergillus spores which led to pulmonary infection with subsequent dissemination to the CNS. Importantly, he did not have overt environmental risk factors; he rarely ventured outside for activity and worked from home as an engineer. Notably, this patient had a negative galactomannan and beta-D-glucan. A meta-analysis evaluating the accuracy of galactomannan as a diagnostic assay for patients with proven or probable invasive aspergillus found a pooled sensitivity of 61% [9]. When the authors limited the analysis to only studies of patients with hematologic malignancies, serum galactomannan had a pooled sensitivity of 58% in patients with proven or probable invasive aspergillus [9]. A meta-analysis evaluating the sensitivity of beta-D-glucan in hematologic malignancy patients found the pooled sensitivity to be 62% [10]. Hence, it is important to interpret noninvasive fungal markers in the context of other clinical, radiographic and microbiologic data. To our knowledge, we present the first case of CNS aspergillosis in a patient on ibrutinib who had not received prior chemo or biologic therapy for a chronic lymphoid malignancy. This case adds to the accumulating body of evidence that ibrutinib carries a substantial increased risk of invasive Aspergillus. It also raises the question as to whether antifungal prophylaxis should be administered concomitantly to patients on ibrutinib or perhaps to a subset of those who are deemed to be at substantially increased risk of invasive fungal infection. This question has indeed been raised before and is nuanced in that antifungal inhibition of cytochrome P450 isoenzyme CYP3A4can raise levels of ibrutinib [11]. Studies are needed to identify patients on ibrutinib who are at greatest risk for invasive fungal infection. Key References: 1. Patterson, T.F., et al., Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis, 2016. 63(4): p. e1-e60. 2. Ruchlemer, R., et al., Ibrutinib associated invasive fungal diseases in patients with CLL and non-Hodgkin lymphoma: an observational study. Mycoses, 2019. 3. Lionakis, M.S., et al., Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell, 2017. 31(6): p. 833-843.e5. 4. Ghez, D., et al., Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood, 2018. 131(17): p. 1955-1959. 5. Margalit, A. and K. Kavanagh, The innate immune response to Aspergillus fumigatus at the alveolar surface. FEMS Microbiol Rev, 2015. 39(5): p. 670-87. 6. Herbst, S., et al., Phagocytosis-dependent activation of a TLR9-BTK-calcineurin-NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med, 2015. 7(3): p. 240-58. 7. Bercusson, A., et al., Ibrutinib blocks Btk-dependent NF-kB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood, 2018. 132(18): p. 1985-1988. 8. Blez, D., et al., Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica, 2019. 9. Pfeiffer, C.D., J.P. Fine, and N. Safdar, Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis, 2006. 42(10): p. 1417-27. 10. Lamoth, F., et al., beta-Glucan antigenemia assay for the diagnosis of invasive fungal infections in patients with hematological malignancies: a systematic review and meta-analysis of cohort studies from the Third European Conference on Infections in Leukemia (ECIL-3). Clin Infect Dis, 2012. 54(5): p. 633-43. 11. Woyach, J.A., Ibrutinib and Aspergillus: a Btk-targeted risk. Blood, 2018. 132(18): p. 1869-1870. |