|
DrFungus.Org
Case of the Month
|
|
Title: Unilateral vision loss in a previously healthy patient Submitted by: Sarah Tran MD, Luke Chang MD Institution: Medical College of Georgia/Augusta University Email: satran@augusta.edu Date Submitted: 09/27/2019 |
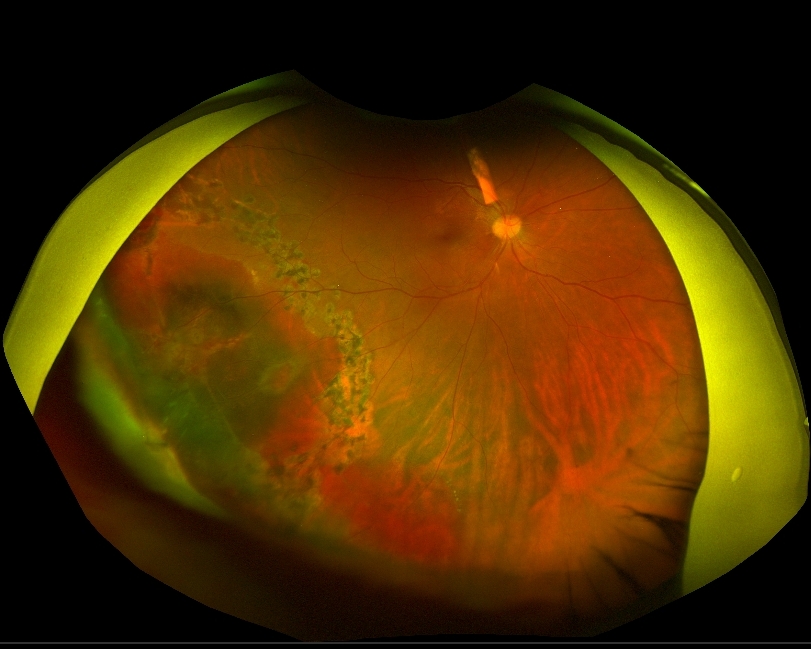
| History of Present Illness: A 30 year-old man presented to the emergency department with 3 weeks of progressive right eye pain and redness, accompanied by blurred and darkened vision in the affected eye. He denied any preceding trauma to the eye, fever, headache, or facial pain. He otherwise felt systemically well. He was initially diagnosed with uveitis and prescribed prednisolone and cyclopentolate eye drops by an outside provider. However, he felt that his symptoms were not improving and presented to a different ophthalmology office for a second opinion. At that time, he was found to have significant anterior chamber inflammatory reaction, vitreous haze, and a fluffy white lesion overlying the peripheral retina of the right eye. After discussing these findings with the patient, he admitted to intravenous (IV) drug use, raising concern for endogenous endophthalmitis. He subsequently underwent vitreous tap and injection of vancomycin, ceftazidime, and amphotericin B. He was also placed on empiric systemic vancomycin, ceftazidime, and amphotericin B for three weeks as an inpatient with little to no improvement in symptoms, despite a second vitreous tap and injection. Given this prolonged course with no improvement, a pars plana vitrectomy was performed. Medical History: None Medications: None Family History: Heart disease in several grandparents. Social History: Denied tobacco use, recent travel, or pets at home. He lives with his girlfriend. Active IV heroin use. Several alcoholic drinks per week. Physical Exam Temp: 36.9°C Pulse: 75 BP: 122/65 SpO2: 97% on room air BMI 28.7 kg GEN: Well appearing young man in no acute distress HEENT: Dilated R pupil with irregular borders and poor light reactivity. Extraocular movements intact. Limited R sided visual acuity at any distance. RESP: Clear bilaterally CV: Regular rhythm, normal rate. No murmurs. SKIN: Warm and dry. Neuro: Alert and interactive without gross deficit. Laboratory Examination: CBC: WBC 7.3, Hgb 12.9 Plt 215 BMP: Na 139 K 4.0 Cl 101 CO2 30 BUN 4 Cr 0.86 Glucose 100 ANA: 1:320 (speckled and homogenous pattern) Rheumatoid factor: <10 IU/mL ACE: 61 u/L (Normal range 8-53) HLA B27: Absent 1,3 B-D Glucan: 151 pg/mL (Normal range < 80) RPR: Nonreactive FTA-ABS: Nonreactive Toxo IgM/IgG: Negative Urine drug screen: Positive for oxycodone Microbiology: Vitreal cultures: 2 samples negative, separated by two weeks Blood cultures: Negative, drawn every 2-3 days over a three-week period Imaging: Chest XR: No acute cardiopulmonary process. Sacroiliac XR: No evidence of ankylosing spondylitis or sacroiliitis. Transthoracic echocardiogram: No identified vegetation or patent foramen ovale. Transesophageal echocardiogram: Small mobile echodensity most likely representing redundant chordae tendinae. Unable to exclude vegetation. |
| Question 1: What are probable/possible diagnoses? A large vitreous opacity–presumed fungus ball—overlying the inferotemporal retina was removed during vitrectomy and sent for cultures. This revealed a large full-thickness retinal hole with surrounding sub-retinal hemorrhage, suggestive of a necrotic process. The area was surrounded with barrier laser to reduce risk of subsequent retinal detachment. A third round of intravitreal vancomycin, ceftazidime, and amphotericin B injections were administered at time of surgery. Culture of the fungus ball: rare Candida tropicalis. Fluconazole MDIL 4 Amphotericin MDIL 0.5 Question 2: What treatment is recommended in the care of this patient? Outcome and treatment: The patient was transitioned to oral fluconazole 800mg daily on discharge and continued on prednisolone, atropine, and moxifloxacin eye drops, as well tobramycin eyedrops. His vision began to improve after the vitrectomy. Fluconazole was continued to complete approximately 16 weeks of total systemic antifungal therapy. Discussion: Ocular candidiasis is a relatively rare, though significant, complication of candidemia. Disease progression involves initial seeding of the choroid, with development of subsequent vitritis, the latter of which is often specifically distinguished as endophthalmitis. A multi-center review of patients with candidemia revealed 16% of patients with ocular involvement, yet only 1.6% with endophthalmitis. 1 Known risk factors for both candidemia and development of candida endophthalmitis overlap: these include presence of indwelling intravenous catheter, recent hospitalization, immunosuppression, diabetes and IV drug use. However, patients who go on to develop endophthalmitis tend to have a longer duration of candidemia. 1 2 Visual symptoms of endogenous endophthalmitis are often insidious in onset. They include decreased visual acuity, eye pain and redness. Diagnosis is usually made by fundoscopic exam findings, which reveal white chorioretinal infiltrates as well as vitreous haze and opacities; marked conjunctival injection and hypopyon are common features on anterior segment exam. Occasionally, the degree of vitritis is significant enough to obscure visualization of the retina, which may also lead to delay in diagnosis. In outpatients presenting with visual symptoms alone, they may be initially misdiagnosed with a noninfectious uveitis. 3 While endogenous candidal endophthalmitis by definition requires hematogenous seeding of fungal organisms, a positive blood culture result for candida is not always identified. This is often secondary to transient candidemia at the time of seeding, especially in the setting of IV drug use. In a review of infectious endophthalmitis cases due to IV drug use, over half of culture-positive cases were due to fungi.4 Fungal endophthalmitis is predominantly caused by candida albicans, though other species of candida have also been identified to a lesser extent. 2 Vitreal cultures are often negative, potentially secondary to selective sampling or early initiation of systemic antifungals. In these cases, early evaluation with non-culture based diagnostics such as serum 1,3 beta-D glucan are often beneficial in guiding empiric therapy. One review estimates the sensitivity and specificity for invasive fungal infection as 76.8% and 85.3%, respectively. 5 However, a majority of cultures from vitrectomy are positive even when performed after an initial vitreous tap and inject along with systemic antifungals. 4 Early initiation of antifungals is associated with improved clinical outcomes. Per the updated 2016 IDSA guidelines for candidiasis, the treatment of candida chorioretinitis with vitritis consists of systemic fluconazole or liposomal amphotericin B as well as intravitreal amphotericin B deoxycholate or voriconazole. Choice of antifungal agent should be guided by organism susceptibilities, being aware of better azole penetration into the vitreous and the relative toxicity of amphotericin B. Echinocandins have demonstrated decent choroid penetration but limited levels in the vitreous.6 Vitrectomy should also be considered to decrease fungal burden. Total systemic antifungal duration should be at minimum 4-6 weeks, guided by improvement in findings as seen on retinal exam. 7 Endogenous candida endophthalmitis remains a rare finding, even in the setting of identified candidemia in an inpatient setting. In this otherwise well-appearing outpatient, obtaining a detailed history including the IV drug abuse in this patient was critical in making an early diagnosis, as there was limited laboratory or culture-data with which to support the diagnosis otherwise. Maintaining high clinical suspicion for the diagnosis allowed for early empiric antifungals with good vitreal penetration while awaiting definitive microbiologic results to better guide long-term therapy. References: 1. Oude Lashof AM, Rothova A, Sobel JD, et al. Ocular manifestations of candidemia. Clin Infect Dis 2011;53(3):262-8. doi: 10.1093/cid/cir355 [published Online First: 2011/07/19] 2. Lingappan A, Wykoff CC, Albini TA, et al. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. Am J Ophthalmol 2012;153(1):162-6 e1. doi: 10.1016/j.ajo.2011.06.020 [published Online First: 2011/09/16] 3. Schiedler V, Scott IU, Flynn HW, Jr., et al. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. Am J Ophthalmol 2004;137(4):725-31. doi: 10.1016/j.ajo.2003.11.013 [published Online First: 2004/04/03] 4. Modjtahedi BS, Finn AP, Papakostas TD, et al. Intravenous Drug Use-Associated Endophthalmitis. Ophthalmol Retina 2017;1(3):192-99. doi: 10.1016/j.oret.2016.10.013 [published Online First: 2017/01/01] 5. Karageorgopoulos DE, Vouloumanou EK, Ntziora F, et al. beta-D-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 2011;52(6):750-70. doi: 10.1093/cid/ciq206 [published Online First: 2011/03/04] 6. Durand ML. Bacterial and Fungal Endophthalmitis. Clin Microbiol Rev 2017;30(3):597-613. doi: 10.1128/CMR.00113-16 [published Online First: 2017/03/31] 7. Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;62(4):e1-50. doi: 10.1093/cid/civ933 [published Online First: 2015/12/19] |