Title: Curvularia alcornii aortic pseudoaneurysm following aortic valve replacement: case report and review of the literature
Submitted by: Dr. Shanti Narayanasamy, M.D., Dr. John Perfect M.D.
Institution: Duke University
Email: shanti.narayanasamy@duke.edu
Date Submitted: 1st September 2020
History: A 53-year-old male with hypertension and hypercholesterolemia underwent tissue aortic valve replacement for bicuspid aortic valve. Six months later, he presented to an outside hospital with sudden acute abdominal pain and lower extremity pain and weakness. He reported a four-month history of right neck pain, and two months of low-grade fevers, sweats, malaise and dysphagia. He was born in Mexico and migrated to the United States 14 years prior. He lived in an urban area of South Carolina and worked as a landscaper.
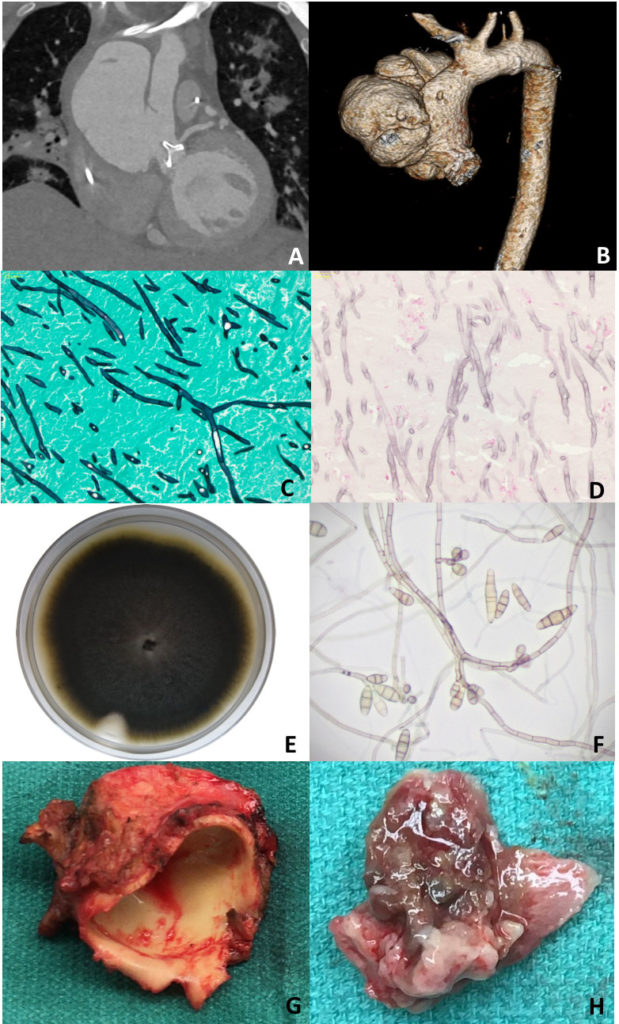
Computed tomography angiogram demonstrated a large multi-lobar ascending aortic pseudoaneurysm (8 x 7.6 cm) causing mass effect on the left atrium and superior vena cava (Figure 1A, 1B), and multiple embolic occlusions of the aorta, renal arteries, superior mesenteric artery, hepatic artery, and splenic artery. Transthoracic echocardiogram showed a large ascending aortic pseudoaneurysm and a well-seated prosthetic aortic valve with no peri-valvular regurgitation. Magnetic Resonance Imaging of the brain showed multiple supra and infratentorial acute infarcts, intra-parenchymal and subarachnoid hemorrhages.
Due to limb ischemia, he underwent emergency laparotomy for surgical thrombectomies of the superior mesenteric and aorto-iliac arteries and bilateral lower limb fasciotomies. The surgical team made note of ‘fungal balls’ within the vascular tree. All material was sent for histopathology and culture.
Physical Examination: On admission: febrile (101°F), heart rate 113/min , blood pressure 188/95 mmHg; other vital signs were within normal limits. Bilateral lower extremities were cold and femoral pulses were absent.
Laboratory Examination: WBC 16,000/ml, platelet count 59,000/ml, hemoglobin 7.4 gm/dl, serum creatinine 2.3 mg/dl, AST 180 U/l, ALT 85 U/l, bilirubin 1.2 mg/dl. Blood cultures: no growth at 5 days. Aortic thrombus bacterial culture: no growth at 3 days.
Question 1: What are probable/possible diagnoses?
The initial microbiologic etiology of the infection was suspected to be bacterial, such as Staphylococcus aureus, streptococci, or gram negative pathogens typical of prosthetic valve endocarditis and mycotic pseudoaneurysm. However, concerns were raised when the blood and the aortic thrombus cultures were negative after five days of incubation.
Microbiology/Diagnostic Tests Performed:
Histopathology of the aortic thrombus showed an organizing thrombus, extensively infiltrated by pigmented septate fungal hyphae (Figure 1C). The Fontana-Masson stain confirmed the presence of melanin in the fungal cell walls (Figure 1D).
The Karius™ test, a next-generation sequencing test designed to detect circulating microbial cell-free DNA in plasma, was sent. No pathogen was detected above the statistical threshold. However, Curvularia species was detected in abundance in the raw data below the statistical threshold.
Eventually, the fungal cultures of the aortic thrombus grew “black colonies” after ten days (Figure 1E). Microscopic examination showed brown, narrow-angle branching, septate hyphae with sympodial growth and brown multi-septate conidia (Figure 1F).
Final Diagnosis: DNA sequencing analysis identified the organism as Curvularia alcornii. The nucleotide sequence of the isolate (GenBank accession number MT658129.1) was independently determined by the Duke Mycology Research Laboratory.
Question 2: What treatment is recommended in the care of this patient?
Treatment: There are few data to guide treatment for Curvularia infection in the literature. Susceptibility testing revealed the following Minimal Inhibitory Concentrations (MICs; micrograms/milliliter): amphotericin B 0.06, voriconazole 0.25, fluconazole 8, itraconazole </= 0.03, ketoconazole 0.125.
The patient received liposomal amphotericin B (L-AmB) 5mg/kg once daily for 12 weeks, micafungin for 2 weeks, and IV/PO voriconazole; all three drugs were commenced at the time the diagnosis of an invasive fungal infection was suspected. Therapeutic levels of voriconazole were not able to be obtained and he was changed to PO itraconazole suspension 300mg two times per day, a therapeutic level (2.3µg/mL) was documented at week 3 of treatment.
Outcome: On Day 21 he underwent an ascending aorta and hemi-arch replacement graft and biological Bentall aortic root and valve replacement. Intra-operative transesophageal echocardiogram showed a large mycotic aneurysm (8.8 x 8cm) (Figure 1G) with fungal balls noted within the aneurysm (Figure 1H). Curvularia alcornii was isolated from fungal cultures of the intra-operative tissue.
He recovered well from the operation. On Day 60 he noted right neck swelling and increased pulsatility. CT angiogram of the neck showed a right external carotid artery pseudoaneurysm. He underwent an open right external carotid artery ligation on Day 83. Cultures of the carotid artery were not sent, but histopathology of the artery demonstrated septate hyphae.
Discussion: To our knowledge, this is the first report of a mycotic aortic aneurysm caused by Curvularia alcornii [1], and only the third reported case of cardiac infection caused by Curvularia species. All three cases were associated with aortic valve replacement and involved the aortic root and presented in an immunocompetent host.
The presentation of the current patient bears striking similarity to the presentation of the two other descriptions of Curvularia endocarditis. Kaufman described a patient who underwent aortic homograft placement six months prior to presentation with mycotic aneurysms of the aorta, spleen, kidneys and brain [2]. Curvularia geniculata was isolated from pre and post-mortem tissue. Bryan described a patient with three months of infective symptoms following an aortic valve replacement six months prior, and was diagnosed with a Curvularia lunata prosthetic valve endocarditis and aortic mycotic aneurysm [3]. Similar to the other two cases, our patient did not have manifestations of disease beyond the vascular tree. No evidence to propose a cutaneous or sino-pulmonary exposure was found, which would have suggested an alternative exposure to the pathogen from his occupation as a landscaper.
Curvularia species have been cultured from the surfaces and air of clinical environments, including operating rooms [4, 5]. A lung transplant recipient developed Curvularia lunata pleural and pericardial infection following a prolonged period with an open chest and multiple chest cavity explorations, however environmental sampling of the ICU did not reveal Curvularia [6]. Levi described a lung transplant patient who developed Curvularia infection of the sternal wound extending to the lung parenchyma, pleural space, and pericardium [7]. Another report described a neonatal sternal wound infection with Curvularia lunata following cardiac surgery for congenital heart disease complicated by delayed chest closure [8]. In all three cases, the infection was fatal.
In 2000, an outbreak of Curvularia contamination of breast implants in five patients who underwent implant surgery at a medical facility was investigated and linked to the sterile normal saline used in the surgical environment [9].
The clinical presentation of this case and literature review suggest that environmental contamination at the time of valve replacement surgery is a possible source of our patient’s Curvularia infection.
Key References:
1. Manamgoda D. S. CL, McKenzie E. H. C., Chukeatirote E. & Hyde K. Two new Curvularia species from northern Thailand. Sydowia 2012; 64(2): 255-66.
2. Kaufman SM. Curvularia endocarditis following cardiac surgery. Am J Clin Pathol 1971; 56(4): 466-70.
3. Bryan CS, Smith CW, Berg DE, Karp RB. Curvularia lunata endocarditis treated with terbinafine: case report. Clin Infect Dis 1993; 16(1): 30-2.
4. Eslami A, Karimi F, Karimi Z, Rajabi Z. A Survey of the quantity and type of biological aerosols in selected wards of a teaching hospital in Ghazvin. Electron Physician 2016; 8(4): 2281-5.
5. Goncalves CL, Mota FV, Ferreira GF, et al. Airborne fungi in an intensive care unit. Braz J Biol 2018; 78(2): 265-70.
6. Rolfe R, Jr., Schell WA, Smith B, Klapper J, Perfect JR, Messina JA. Black mold takes hold and story told. Med Mycol Case Rep 2020; 29: 12-4.
7. Levi M, Basgoz N. Fungal wound infection in a lung transplant recipient. Transpl Infect Dis 2000; 2(1): 36-43.
8. Yau YC, de Nanassy J, Summerbell RC, Matlow AG, Richardson SE. Fungal sternal wound infection due to Curvularia lunata in a neonate with congenital heart disease: case report and review. Clin Infect Dis 1994; 19(4): 735-40.
9. Kainer MA, Keshavarz H, Jensen BJ, et al. Saline-filled breast implant contamination with Curvularia species among women who underwent cosmetic breast augmentation. J Infect Dis 2005; 192(1): 170-7.
