(described by Micheli ex Link in 1809)
Taxonomic Classification
Kingdom: Fungi
Phylum: Ascomycota
Order: Eurotiales
Family: Trichocomaceae
Genus: Aspergillus
Description and Natural Habitats
Aspergillus is a filamentous, cosmopolitan and ubiquitous fungus found in nature. It is commonly isolated from soil, plant debris, and indoor air environment. While a teleomorphic state has been described only for some of the Aspergillus spp., others are accepted to be mitosporic, without any known sexual spore production.
Species
The genus Aspergillus includes over 185 species. Around 20 species have so far been reported as causative agents of opportunistic infections in man. Among these, Aspergillus fumigatus is the most commonly isolated species, followed by Aspergillus flavus and Aspergillus niger. Aspergillus clavatus, Aspergillus glaucus group, Aspergillus nidulans, Aspergillus oryzae, Aspergillus terreus, Aspergillus ustus, and Aspergillus versicolor are among the other species less commonly isolated as opportunistic pathogens. See the list of obsolete names and synonyms for older names of these species.
Pathogenicity and Clinical Significance
Aspergillus spp. are well-known to play a role in three different clinical settings in man: (i) opportunistic infections; (ii) allergic states; and (iii) toxicoses. Immunosuppression is the major factor predisposing to development of opportunistic infections [1048]. These infections may present in a wide spectrum, varying from local involvement to dissemination and as a whole called aspergillosis. Among all filamentous fungi, Aspergillus is in general the most commonly isolated one in invasive infections. It is the second most commonly recovered fungus in opportunistic mycoses following Candida.
Almost any organ or system in the human body may be involved. Onychomycosis, sinusitis, cerebral aspergillosis, meningitis, endocarditis, myocarditis, pulmonary aspergillosis, osteomyelitis, otomycosis, endophthalmitis, cutaneous aspergillosis, hepatosplenic aspergillosis, as well as Aspergillus fungemia, and disseminated aspergillosis may develop [124, 567, 638, 748, 800, 811, 817, 839, 874, 938, 952, 1161, 1536, 1975]. Nosocomial occurrence of aspergillosis due to catheters and other devices is also likely [1386]. Construction in hospital environments constitutes a major risk for development of aspergillosis particularly in neutropenic patients [1363].
Aspergillus spp. may also be local colonizers in previously developed lung cavities due to tuberculosis, sarcoidosis, bronchiectasis, pneumoconiosis, ankylosing spondylitis or neoplasms, presenting as a distinct clinical entity, called aspergilloma [852, 1056]. Aspergilloma may also occur in kidneys [974].
Some Aspergillus antigens are fungal allergens and may initiate allergic bronchopulmonary aspergillosis particularly in atopic host [829, 1271]. Some Aspergillus spp. produce various mycotoxins. These mycotoxins, by chronic ingestion, have proven to possess carcinogenic potential particularly in animals. Among these mycotoxins, aflatoxin is well-known and may induce hepatocellular carcinoma. It is mostly produced by Aspergillus flavus and contaminates foodstuff, such as peanuts [1576].
Aspergillus spp. can cause infections in animals as well as in man. In birds, respiratory infections may develop due to Aspergillus. It may induce mycotic abortion in the cattle and the sheep [2144]. Ingestion of high amounts of aflatoxin may induce lethal effects in poultry animals fed with grain contaminated with the toxin.
Since Aspergillus spp. are found in nature, they are also common laboratory contaminants.
The major macroscopic features remarkable in species identification are the growth rate, color of the colony, and thermotolerance [462, 1295, 2144].
Except for Aspergillus nidulans and Aspergillus glaucus, the growth rate is rapid to moderately rapid. While Aspergillus nidulans and Aspergillus glaucus grow slowly and reach a colony size of 0.5-1 cm following incubation at 25°C for 7 days on Czapek-Dox agar, those of the remaining species are 1-9 cm in diameter in the specified setting. These variations in growth rate help in species identification.
Aspergillus colonies are downy to powdery in texture. The surface color may vary depending on the species. The reverse is uncolored to pale yellow in most of the isolates. However, reverse color may be purple to olive in some strains of Aspergillus nidulans and orange to purple in Aspergillus versicolor (TABLE 1).
Aspergillus fumigatus is a thermotolerant fungus and grows well at temperatures over 40°C. This property is unique to Aspergillus fumigatus among the Aspergillus species. Aspergillus fumigatus can grow at a temperature range of 20 to 50 °C.
TABLE 1. The color of the colony in various Aspergillus species
| SPECIES | SURFACE | REVERSE |
|---|---|---|
| A. clavatus | Blue-green | White, brownish with age |
| A. flavus | Yellow-green | Goldish to red brown |
| A. fumigatus | Blue-green to gray | White to tan |
| A. glaucus group | Green with yellow areas | Yellowish to brown |
| A. nidulans | Green, buff to yellow | Purplish red to olive |
| A. niger | Black | White to yellow |
| A. terreus | Cinnamon to brown | White to brown |
| A. versicolor | White at the beginning, turns to yellow, tan, pale green or pink | White to yellow or purplish red |
Microscopic Features
The basic microscopic morphology is same for all species. However, some other microscopic structures are unique to certain species and constitute the key features for species identification together with the surface color of the colony (TABLE 2)[462, 1295, 2144].
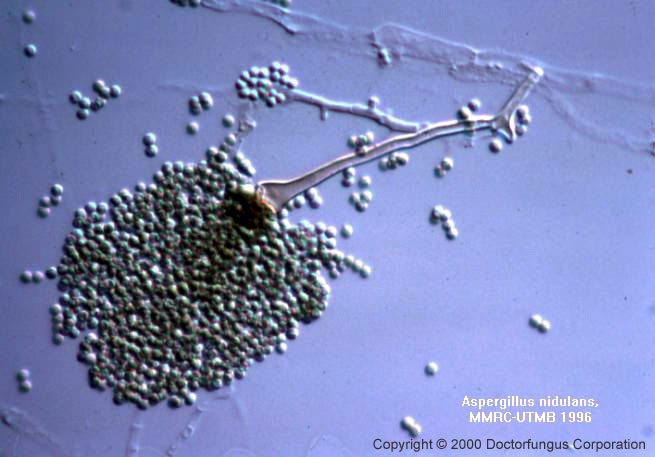
1. COMMON TO ALL SPECIES: Hyphae are septate and hyaline. The conidiophores originate from the basal foot cell located on the supporting hyphae and terminate in a vesicle at the apex. Vesicle is the typical formation for the genus Aspergillus. The morphology and color of the conidiophore vary from one species to another. Covering the surface of the vesicle entirely (“radiate” head) or partially only at the upper surface (“columnar” head) are the flask-shaped phialides which are either uniseriate and attached to the vesicle directly or are biseriate and attached to the vesicle via a supporting cell, metula. Over the phialides are the round conidia (2-5 µm in diameter) forming radial chains.
2. UNIQUE TO CERTAIN SPECIES ONLY: Other microscopic structures include sclerotia, cleistothecia, aleuriconidia, and Hulle cells. These structures are of key importance in identification of some Aspergillus species. Cleistothecium is a round, closed structure enclosing the asci which carry the ascospores. The asci are spread to the surrounding when the cleistothecium bursts. Cleistothecium is produced during the sexual reproduction stage of some Aspergillus species. Aleuriconidium is a type of conidium produced by lysis of the cell that supports it. The base is usually truncate and carries remnants of the lysed supporting cell. These remnants form annular frills at its base. Hulle cell is a large sterile cell bearing a small lumen. Similar to cleistothecium, it is associated with the sexual stage of some Aspergillus species.
TABLE 2. Microscopic features of various Aspergillus species
| SPECIES | CONIDIOPHORE | PHIALIDES | VESICLE | S | C | HC | A |
|---|---|---|---|---|---|---|---|
| A. clavatus | Long, smooth | Uniseriate | Huge, clavate-shaped | – | – | – | – |
| A. flavus | Colorless, rough | Uni-/biseriate | Round, radiate head | + (In some strains, brown) | – | – | – |
| A. fumigatus | Short (<300 µm), smooth, colorless or greenish | Uniseriate | Round, columnar head | – | – | – | – |
| A. glaucus group | Variable length, smooth, colorless | Uniseriate | Round, radiate to very loosely columnar head | – | + (yellow -orange) | – | – |
| A. nidulans | Short (<250 µm), smooth, brown | Biseriate, short | Round, columnar head | – | + (red) | + | – |
| A. niger | Long, smooth, colorless or brown | Biseriate | Round, radiate head | – | – | – | – |
| A. terreus | Short (<250 µm), smooth, colorless | Biseriate | Round, compactly columnar head | – | – | – | + (solitary, round, produced directly on hyphae) |
| A. versicolor | Long, smooth, colorless | Biseriate | Round, loosely radiate head | – | – | + (in some strains) | – |
S: Sclerotia
C: Cleistothecia
HC: Hulle cells
A: Aleuriconidia
Histopathologic Features
See our histopathology page.
Compare to
Syncephalastrum at first look may resemble Aspergillus niger. The absence of phialides and the presence of tubular sporangia in Syncephalastrum isolates are noticed by careful examination. In contrast to Aspergillus, the hyphae of Syncephalastrum are nonseptate [1295].
Laboratory Precautions
No special precautions other than general laboratory precautions are required.
Susceptibility
Following the documentation of the NCCLS proposed standard (M38-P) for in vitro susceptibility testing of filamentous fungi [1622], more data have been available on in vitro activity of Aspergillus spp. Although the MIC breakpoints for the available antifungal agents are not defined yet, the results of these studies are in consensus for a number of features. The MICs obtained for different species of Aspergillus are in general similar. While most of the Aspergillus isolates yield acceptably low MICs for amphotericin B, itraconazole, and voriconazole, high MICs potentially predictive of resistance have been reported for a number of isolates [119, 1130, 1669]. Among these are, amphotericin B MICs for Aspergillus terreus [2203]. A few itraconazole-resistant Aspergillus fumigatus isolates have also been identified [578]. Significantly and finally, voriconazole appears effective in vitro against itraconazole-resistant Aspergillus fumigatus [119].
The in vitro activity of novel antifungal agents, such as the echinocandins are also of current interest. Although the visualization of the in vitro effect of echinocandins requires distinctive parameters (MEC; minimum effective concentration), they are active against Aspergillus both in vitro and in vivo [120, 1406, 1670]. The recent demonstration of the synergistic effect of amphotericin B with echinocandins against Aspergillus in vitro and in animal models is noteworthy and exciting [2159](Arikan, ICAAC 2000).
Correlation of the in vitro susceptibility test results with the clinical outcome has been documented for itraconazole and Aspergillus [573]. Treatment of invasive aspergillosis is still troublesome with high rate of mortality. While amphotericin B (including its lipid formulations) and itraconazole are the currently available therapeutic options, the clinical success rate is still unsatisfactory due both to the low efficacy and/or high toxicity of the drugs and existence of unfavorable immune status of the host, such as lack of recovery from neutropenic state [568, 569, 572, 608, 659, 740, 830, 910, 958]. The concommitant use of colony stimulating factors may activate the macrophages, enhance their fungicidal activity and prevent dissemination of the infection [780].
The novel azoles (e.g., voriconazole, posaconazole, or ravuconazole), glucan synthesis inhibitors (e.g., caspofungin, N/A(L):V-echinocandin, FK463) and liposomal nystatin are active in vitro against Aspergillus and remain promising for future therapy of aspergillosis [122, 579, 683, 890, 1091, 1129, 1152, 1434, 1569, 2352].
For MICs of various antifungal drugs for Aspergillus, see our N/A(L):susceptibility database.