Overview
An evaluation for the presence of indoor fungi in a residence or other building usually has one of two broad goals. (Note: Indoor fungus problems are almost exclusively due to mould fungi rather than yeast fungi, and the subsequent discussion will focus on the moulds.) First, structural damage may follow from mould growth on (or in) walls and other areas in the home. When moulds have grown on walls, they may have also grown through the wallboard into the internal space of the wall. Mould growth on carpets and other items in a room can concurrently occur. The moulds associated with indoor environment problems grow on damp to wet materials containing cellulose and other sources of nutrients.
The second concern regarding indoor moulds is their potential to cause human disease. Infection is unlikely unless the exposed person has very reduced functioning of the immune system (e.g., is on long-term therapy with high doses of corticosteroids). The diseases caused by indoor fungi can be either allergic, toxic, or a combination of both.
To determine if a fungus problem exists, sampling is done both indoors and outdoors. The idea is that the amount of fungus indoors is equal to, or less than what is outside. Assessment for environmental moulds involves two broad approaches that we discuss in turn below. First, one can look for (A):moulds growing on or within household furnishings or the building materials used to construct the building. In this search, one can examine the content of vacuum cleaner bags, collect swipe specimens from surfaces, or cut out and examine pieces of material (this is called bulk sampling) that have obvious fungal growth (also sometimes referred to as mildew). This is usually the most useful type of search.
Second, one can (A):examine the air for its content of moulds. A general estimate of the number and different types of mould particles (spores and conidia) in the air can be made by microscopic examination of particles impacted upon a filter or coated microscope slide. Alternatively, the fungi in the air may be impacted upon a growth medium. After being grown in the laboratory, this method permits an accurate identification of the viable fungi that were in the air when the sample was taken.
The big confusion in both settings is that moulds are simply everywhere, and random sampling will almost always turn up at least a few colonies of moulds. Thus, before sampling you always want to think about what you’ll do with the results. You must always keep in mind that we do not live in sterile conditions. Moulds that aren’t visible and that aren’t growing are usually not a problem! Most situations do not require use of any of the techniques on this page beyond the look and sniff approaches discussed in the next section.
Searching for Moulds on Surfaces
A visual and olfactory (look and smell) inspection is the most important step in identifying a possible contamination problem [90, 93, 96]. You are done with cleanup when (a) there is no visible mould, (b) there are no mould odors, and (c) you have fixed the moisture/water problem so that it will not recur [95].
If sampling of surfaces needs to be done, there are several possible strategies:
- If you see a stain that might be mould, you can put a piece of transparent tape on the stain, pull the tape off, and then inspect whatever sticks to the tape for the presence of fungal fragments.
- You can swab the surface and culture the collected material.
- You can vacuum the surface and then inspect the contents of the vacuum cleaner bag.
- You can cut out a piece of the surface or material and either culture it or inspect it microscopically.
These strategies are all pretty straightforward, and there’s not a lot of unusual technology involved.
Searching for Moulds in the Air
Indoor air quality (IAQ) can be measured many ways:
- Carbon Monoxide (CO) in non-industrial facilities is found at 1.2 to 4.2 ppm (parts per million). The National Ambient Air Quality Standard for outside are is 9 ppm for an 8 hour exposure and 35 ppm for a one-hour exposure.
- Carbon Dioxide is a general indicator of IAQ. Levels greater than 800-1000 ppm are often accompanied by complaints about air quality. Carbon dioxide itself is not the problem, but is rather a marker of other pollutants that accumulate along with it [1883].
- Temperate and Relative Humidity should be ~70-75F and 30-60% (or, even better, 30-50%).
- Particulate control is a function of the filtering of the air.
Of these, particulate control and analysis is the measure that is relevant to the search for moulds in the air. But, before you do any measurements, it is important to review the key concept of the indoor:outdoor ratio. In short, the air inside the building must be compared to the air outside the building. If the building system is removing 99% of the particles but the air outside is incredibly dirty, the air inside will be 99% less dirty but that doesn’t mean it will be sparkling clean. In general, a ratio of 1:3 (that is, a 2/3rd reduction) is acceptable in most commercial buildings.
So, now that you’re ready to do some measurements, there are three key tools:
Particle Counts. By using a hand-held particle counter, one can obtain instantaneous particle readings. It is usual to focus on particles > 0.5 microns in size. On a typical day, one might see ~300,000 such particles/cu. ft. in the outside air. Non-medical commercial buildings might show counts of ~100,000 particles/cu. ft. Inside a well-filtered hospital, one might then expect to see < 50,000 particles/cu. ft. in general care areas and < 10,000 particles/cu. ft. in highly filtered (e.g., HEPA filtered) areas. Extremely clean rooms can achieve particle counts as low as 1,000 particles/cu. ft., but this requires careful control of both the air-handling equipment and the objects (and people) in the environment.
Of importance, the infectious bacterial and fungal particle are generally a good deal larger than this. For example, the spherical conidia of Aspergillus spp. are 2-5 microns in diameter. Thus, particles small than about 1 micron in size are probably non-infectious dust particles. Particle counters can be set to count in various particle size ranges. Counting particles all the way down to 0.5 microns (or even 0.3 microns) in size can be a way to assess system function for systems that are capable of delivering highly filtered air (e.g., this might be relevant to a HEPA-filtered area). On the other hand, systems that use the more typical 90-95% filters would best be judged based on somewhat larger particle sizes. (A):Filters used in private residences are typically so low on the performance scale that you would not expect any meaningful reduction in particle counts. You thus need to think a bit about what you want to count and the sorts of results you would expect before turning on that counter!
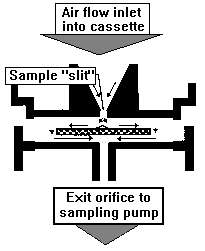
| Particle analysis. So, what are those particles? Well, the fastest way to find out is by microscopic inspection of the particles. The Air-O-Cell™ Cassette is one way to collect them for inspection. It operates by inertial impaction: particles are pulled through the device at 30 to 35 mph, funneled through the tapered slit, and then slammed onto the sticky surface of a special slide that is placed just below the slit (see diagram). The slide is then removed and examined under the microscope.
The fungi are NOT cultured, and non-viable (dead) fungal particles will be counted right along with the viable (living) particles. Thus, the counts generated by this method will usually be higher than those generated by an actual culture of the air. Fungi are identified based on their morphology (shape and color). Sometimes this means that a specific genus can be identified, other times it means that the fungal particle can only be placed within a broad group of fungi that have similar shape and color. Here is an example of the kind of result you might receive. Asp-Pen means Aspergillus/Penicillium-like (the report will combine these two fungi because it not possible to tell them apart by just looking at their conidia). This example shows a nice 1:100 reduction in particle quantity.
|
|
| Although some particles don’t stick well and others may be aerodynamically too light to be captured, this technique does collect about 85% of the particles. And, unlike cultures which take 7-10 days, the Air-O-Cell™ results can be back within a day or two.Of course, there are other ways to sample particles. We have highlighted the Air-O-Cell because of its easy of use in hospital environments. As another example, shown at right is a Burkhard 7-day volumetric spore trap. This device uses a sticky tape concept similar to that of the Air-O-Cell, but combines this with rotation of the tape on a drum. This permits serial sampling for latter analysis. (Image courtesy A. Staresinic. See (E):www.burkard.co.uk for more information on the spore trap.) |
|
Culture. Finally, you can culture the air. Here, you can choose to look for both moulds and bacteria. (Yeasts may also appear in these cultures, but as noted above they are usually not relevant to an environmental evaluation.) What is detected depends on how the measurements are done. A reasonable choice of culture media and subsequent incubation conditions are listed below. Interpeting these results takes a little practice and you might wish to read further on this topic [87, 149, 394, 975, 1700]. Some general guidelines can be offered, but always remember that the key principle is the indoor:outdoor ratio! When you see indoor counts that exceed the outdoor counts, this suggests that growth and release of fungi is occurring somewhere within the indoor environment.
- Bacterial culture medium, incubated at 25°C. This gives you a sense of general building cleanliness. It is common to see mostly gram-positive bacterial skin flora (e.g., Staphylococcus species). The occasional gram-negative rod may be seen, but having much of this suggests fecal contamination. This type of contamination might be due to sewage backup or the presence of rodents or other animals in the building.
- Fungal culture medium, incubated at 25°C. See discussion of next condition.
- Fungal culture medium, incubated at 35°C. Fungi that grow at 35°C are referred to as thermotolerant fungi and are able to produce infections of warm-blooded people and animals. (Note that this means that the fungus could produce an infection, not that it will produce an infection. Infections due to these types of fungi do not usually occur in healthy people, but instead require significant reduction in the function of the immune system.) But, there are many, many more fungi that will only grow at 25°C. These thermo-INtolerant fungi may be 10-fold more plentiful than their thermotolerant cousins. The thermo-INtolerant fungi can certainly grow in or on building materials and produce allergens that make you sneeze. Due to being more plentiful, the proportionate reduction from indoor to outdoor of these fungi is especially easy to measure. The fungi of greatest interest will vary with your measurement goals.
As an example, here are some data that are typical of the kinds of measurements one might see from a well-filtered building. In this example, better than an 80% reduction in microbial burden is being achieved (CFU = colony-forming unit):
| Sample | Bacteria, 25°C |
Fungi, 25°C |
Fungi, 35°C |
| Outside Air | 263 CFU/m3 | 343 CFU/m3 | 93 CFU/m3 |
| Inside Air | 47 CFU/m3 | 24 CFU/m3 | 7 CFU/m3 |
Interpretation of Data
Having sampled, one then has to decide what to do with the results. Again, we come back to the inside:outside ratio. There are no absolute fixed values that are good or bad–everything must be considered as a function of what you expect your building and its air-handling systems to provide. A good discussion of these ideas is found in the text by Macher et al. on bioaerosols [87].
The lack of fixed reference points in this process cannot be overemphasized. To the best of our knowledge, there are no widely accepted standards for what represents a good or bad level of fungus in the environment. None of the publications from the CDC or EPA support any specific cut-off or threshold value. For example, the (E):EPA website as of September 2005
states that “Standards or Threshold Limit Values (TLVs) for airborne concentrations of mold, or mold spores, have not been set. Currently, there are no EPA regulations or standards for airborne mold contaminants.” Where suggestions have been put forth, the suggestions reflect the general absence of data. As an example, a 1995 document from Health Canada on this topic states that ‘more than 50 CFU/cu. meter may be reason for concern if there is only one species present other than Cladosporium or Alternaria.’
Given the absence of well-supported target values, your general goal is to know your equipment and building well enough to know what to expect and then monitor for that result. Check your filters. Are they clean? Are they properly seated and sealed? Is everything actually on? Is your air balance correct? Is the equipment actually of the right capacity for the space that it ventilates? If your building has multiple air handlers or air zones, are the results similar across units or zones of the same design? What do your look-and-smell tests show? Are the occupants of the building complaining of symptoms that might be due to fungi? All of these issues should be considered if you find results that seem aberrant.
Target values of ~0.1 colony forming units (CFU) per cubic meter of Aspergillus spp. and 1 CFU per cubic meter for other (A):thermotolerant fungi have been proposed for hospital environments [2179, 2180], but these recommendations are not broadly endorsed. Recommendations for other environments are not available. The relevance of any such targets to your setting is an important question. Considering just the hospital environment, one might say “Sure, I’d like the air completely fungus-free.” But in looking at the broader picture, one must acknowledge that patients are typically only in the hospital a few days. Before they came in and when they go home, they will be exposed to the kinds of environmental mould concentrations shown above. Unless someone chooses to take up permanent residence in a hospital, it would seem rational to conclude that extremely clean air is relevant only in very specific short-term settings. One example might be a unit that cares for burned patients–due to the loss of the skin barrier, they are at high risk for cutaneous fungal infections. Another example might be a unit that performs high-risk bone marrow transplanation in an in-patient setting. Overall, common sense (which is unfortunately not all that common) must preside!
In our work, we have found that the main use for Air-O-Cell™ and cultures is at the beginning of the use of a building–at the time of its commissioning. We like to tie the baseline results to the local particle counts. Then, subsequent monitoring can focus on particle counts–as long as you are seeing particle counts in the expected range, you can be assured that the equipment is probably functioning correctly. This, along with suitably timed preventive maintenance and filter exchange is the key to producing a steady stream of clean air.
Data from Residential Buildings
The quality of the filtration in most residential buildings is minimal and most residences are principally naturally ventilated. That is, the internal air is functionally equivalent to the outdoor air. The air-handling system serves principally to heat or cool the air–the overall level of filtering is quite low. Indeed, when you open those windows so that you may enjoy that cool summer breeze, you are effectively joining your internal environment to the outdoor environment. Thus, your goal is going to be environmental test results that show that indoor air does not contain more fungi than are found outdoors. It is not reasonable to expect the air to be any cleaner. Thus, the look-and-smell approach to fungal assessment is particularly appropriate for the residential setting.
Summary of Key Ideas
- A visual and olfactory inspection is the most important step in identifying a possible contamination problem [90, 93, 96]. You are done with cleanup when (a) there is no visible mould, (b) there are no mould odors, and (c) you have fixed the moisture/water problem so that it will not recur [95]. In addition, if mould sampling was done, the kinds and concentrations of mould and mould spores in the building should be similar to those found outside [95].
- Although a great deal of attention has been paid to the mould (A):Stachybotrys chartarum, it is only one of many moulds that can (a) produce mycotoxins and (b) produce allergic responses [95, 96]. The most common indoor moulds are Aspergillus, Alternaria, Cladosporium, and Penicillium [96, 745]. Identification of the genus and species of any given fungus requires microscopic techniques and considerable expertise [90]. A repeated theme throughout the EPA documents in particular is that the most practical approach is simply to eliminate all visible mould growths and smells from the environment.
- If bulk or surface sampling is performed (that is, if sections of building material are cut out and cultured or if surface swipes are performed), the presence of few or trace amounts of fungal spores in bulk/surface sampling should be considered background [90]. Amounts greater than this or the presence of fungal fragments (e.g., hyphae or conidiophores) may suggest fungal colonization, growth, and/or accumulation at or near the sampled location [90]. However, the value of bulk sampling appears minimal–the documents repeatedly emphasize the (a) the ubiquitous presence of trace amounts of fungi and (b) the importance of visual assessment for fungal growth.
- Air sampling should entail simultaneous indoor and outdoor sampling [90, 95]. Widely accepted standards or threshold limit values (TLVs) for airborne concentrations of moulds or mould spores have not been set. As of September 2005, there are no EPA regulations or standards for airborne mould contaminants [95] and (E):EPA website. One might expect healthcare facilities to be among the most sensitive of areas in this regard. The 2001 CDC Draft Guideline for Environmental Infection Control in Healthcare Facilities states in recommendation 1-2.8: “No recommendation on routine microbiologic air sampling before, during, or after construction or before or during occupancy of areas housing immunocompromised patients.” The CDC document recommends microbiological sampling only if there is evidence of ongoing transmission of disease(recommendation 1-2.12).As cultures take up to two weeks for results, use of particle counts can provide a rapid assessment of the functional status of the air filtering system. Particle counts are now specifically recommended by the just mentioned CDC draft guideline [83]. Microscopic characterization of airborne particles by the Air-O-Cell™ sampling technique may be a useful additional way to quickly characterize the airborne particles.
- Moisture is required for continued fungal growth [96]. Use of a moisture meter can be very helpful in identifying problem areas [90, 95]. The University of Minnesota has suggested procedures for use of meters.
Other Resources
We provide specific literature references on most of our pages. However, we also have a separate page devoted just to a critical summary of the mould-related literature that is readily found on the web. Check it out!
About These Pages
The material and ideas here are drawn from many sources, including our own experience. However, this is an area with few guidelines and even fewer hard facts. So, you must always apply common sense in choosing how to adapt the ideas presented here to your own situation. When in doubt, please consult with a professional. At times, there is simply no substitute for experience and personal knowledge.