Overview
The term “Invasive Candidiasis” is equivalent to “Disseminated Candidiasis”, “Systemic Candidiasis,” and “Hematogenous Candidiasis”. We preferentially use the first term, but you will see the others used as well. Candida spp. can invade and cause disease in virtually any organ of the body. However, there are many natural barriers to this form of disease, and it only occurs in individuals who have reduced or altered host defenses.
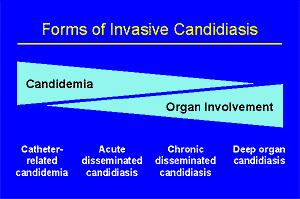 Classifying the forms of invasive candidiasis is a bit of a challenge. The most basic form of invasive candidiasis is candidemia. Actually, it is generally thought that all other forms of invasive candidiasis follow an episode of candidemia. However, the episode of candidemia is not always detected, and thus at times disease of an organ is the first sign of invasive candidiasis. Thus, while some patients present with the dramatic manifestations of sepsis that may accompany an episode of candidemia, others may have never been thought candidemic but subsequently will turn up with focal candidiasis of a deep organ. Understanding this range of manifestations is important. As a way to visualize this, we often think of candidiasis as having four overlapping forms. In this section, we discuss general features of the (A):epidemiology, (A):clinical manifestations, (A):diagnosis, and (A):treatment of invasive candidiasis. You may want also find it useful to review data on the (A):specific types of invasive candidiasis.
Classifying the forms of invasive candidiasis is a bit of a challenge. The most basic form of invasive candidiasis is candidemia. Actually, it is generally thought that all other forms of invasive candidiasis follow an episode of candidemia. However, the episode of candidemia is not always detected, and thus at times disease of an organ is the first sign of invasive candidiasis. Thus, while some patients present with the dramatic manifestations of sepsis that may accompany an episode of candidemia, others may have never been thought candidemic but subsequently will turn up with focal candidiasis of a deep organ. Understanding this range of manifestations is important. As a way to visualize this, we often think of candidiasis as having four overlapping forms. In this section, we discuss general features of the (A):epidemiology, (A):clinical manifestations, (A):diagnosis, and (A):treatment of invasive candidiasis. You may want also find it useful to review data on the (A):specific types of invasive candidiasis.
Epidemiology and Risk Factors
Invasive candidiasis is not a disease seen in normal healthy hosts. Rather, there are a large number of reasonably well characterized risk factors for this group of diseases (see table, below). Some of the risk factors are other diseases (e.g., renal failure & hemodialysis), while others are induced by various therapies (e.g., chemotherapy or gut-related surgery). Despite the association of burn units with relatively high rates of invasive candidiasis [772], this association is otherwise little documented [1305].
The largest single watershed is that of cancer vs. non-cancer settings. Both neutropenia and chemotherapy-induced injury to the gut wall dramatically increase the risk for invasive candidiasis. In the non-cancer setting, catheters, gut-wall surgery, and prematurity are arguably the most significant factors.
| Risk Factors | Cancer Patients | Non-Cancer Patients |
|---|---|---|
| Granulocytopenia, bone marrow transplantation, type/duration of chemotherapy, graft-versus-host disease | [310, 870, 1154, 1922, 1974, 2063, 2433] | n/a |
| Degree of chemotherapy-related mucositis | [310, 1974] | n/a |
| Colonization with Candida | [29, 933, 1154, 1442, 1922, 2021, 2129, 2131] | [325, 1808, 2411] |
| Use of broad-spectrum antibiotics | [1442, 1922, 2063] | [325, 339, 1808, 2000, 2411] |
| Hemodialysis and/or azotemia | [325, 2411] | |
| Central vascular catheters | [1154] | [325, 2000, 2411] |
| Heroin addicts | [247, 633] | |
| Severity of illness | [1808] | |
| Hyperalimentation | [264, 297, 1022, 1397, 2000] | |
| Recurrent or persistent gastrointestinal perforation | [361, 651] | |
| Prior surgery | [233, 264, 339, 695, 791, 993] | |
| Solid-organ transplantation | [216, 395, 2065] | |
| Neonates: Gestational age, low Apgar score, length of stay, shock, H2 blockers, and intubation | [2000] |
Clinical Manifestations
The clinical manifestations of invasive candidiasis are, unfortunately, often completely non-specific. The following may be observed:
- Fever and the Sepsis Syndrome.
Fever is often the only clinical clue of invasive candidiasis on a high risk patient. Likewise, progressive sepsis with multi-organ failure has been described in surgical patients who are developing invasive candidiasis [695]. Thus, persistent unexplained fever or sepsis that is not responding to broad spectrum antibiotics is the setting for the more acute forms of invasive candidiasis. In this regard, there has long been recognition of the need for empirical antifungal therapy in neutropenic patients. And, more recently, the idea that this concept might apply in selected non-neutropenic patients and neonates is receiving increasing attention.
Cutaneous Lesions Consistent with Invasive Candidiasis.
An important group of skin findings in patients affected with invasive candidiasis has been described in cancer patients [272] and heroin addicts [247]. It is possible that these processes represent the same lesion in two immunologically different hosts. However, the data on this are not clear and we will discuss them separately.- Neutropenic patients.
In about 15% of neutropenic patients with invasive candidiasis, a characteristic macronodular rash will appear. The rash may be isolated (extremities, abdomen) or may cover the entire body. These lesions are frequently confused with drug allergies. Biopsy is important, and the pathologist should be made aware of the suspected disease: only a careful examination of the deeper layers of skin, particularly the vascularized areas and the dermis, will show small oval blastopores with buds and pseudohyphaes [272]. - Heroin addicts.
About half of heroin addicts that present with disseminated candidiasis present with a rash [247]. The lesions include papules, pustules, nodules and folliculitis. [242, 362, 655, 1514]. The lesions are commonly encountered in hairy areas, mainly the scalp and beard.
- Neutropenic patients.
- Retinal Lesions of Invasive Candidiasis.
Finally, a significant percentage of candidemic patients will have one or more retinal lesions that may represent candidal endophthalmitis [308, 1254]. The problem with these lesions is that the majority of them are relatively non-specific and may appear similar to lesions caused by diabetes or hypertension. It is, however, important to find and prospectively follow any such lesions in patients with documented candidemia. We strongly encourage a good retinal examination in candidemic patients.On the other hand and despite comments by some authors [2407], we have found retinal examinations to have low sensitivity and to be of very limited value as a diagnostic tool in patients who are at high-risk for disseminated candidiasis, but who lack positive blood cultures for Candida. Both recent guidelines in this area [1915] and the research protocols used in North American [1746] and European [804, 1768, 1808] studies of invasive candidiasis make it clear that such testing is not standard of care.
Specific Diagnostic Strategies
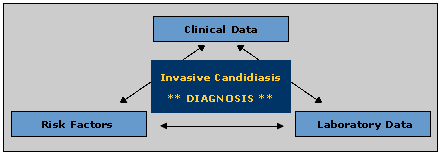
The three components of diagnosing invasive candidiasis
No single test is available to make a straightforward diagnosis on invasive candidiasis. Instead, a physician must combine his or her knowledge of the patient’s risk factors for invasive candidiasis, clinical signs that might suggest candidiasis, and a variety of possible bits of laboratory data. The first two items have been discussed above, and this section will now examine laboratory-based aids to the diagnosis of this disease.
A. Microbiology Data
- Gram stain. N/A(L):Candida spp. appear as Gram-positive 4-6 micrometer, thin-walled, ovoid yeasts. Budding and pseudohyphae may be seen, but are not invariably present.
- Culture. Candida spp. grow easily when present in biopsy specimens, aspirates, or surface cultures of involved sites. Only 1 to 3 days are necessary to grow C. albicans, C. parapsilosis and C. tropicalis on any standard medium, while growth of C. krusei and C. glabrata may take slightly longer [1059, 1188, 1845]. The colonies in general, appear smooth, creamy, and glistening, although some species characteristically produce coarser colonies.
- Recovering Candida from the blood. While candidemia would seem to be a key element of essentially all cases of invasive candidiasis, retrospective studies have shown that blood cultures are positive in less than 50% of patients with autopsy-proven invasive candidiasis. [1948]. The best sensitivity reported to date has been achieved with the lysis-centrifugation method, and even then it was only 58% [226]. While this technique reduces the time needed for Candida spp. and to allow estimation of the number of colonies forming units of that organism per milliliter of blood, it is quite expensive and labor intensive [246, 275]. Other techniques with at least equal sensitivity include the BACTEC high-blood-volume fungal media (HBV-FM) and the BacT/Alert system [1951, 2442].
- Recovering Candida from the urine. Candiduria is common, especially in hospitalized patients having urinary catheters [859, 2448]. Unlike bacteriuria, however, there is no simply way to decide if funguria is clinically relevant. Neither the absolute colony count nor the presence or absence of white blood cells is consistently helpful [1626]. Recent work now strongly suggests that asymptomatic candiduria in the low-risk patient is of little clinical relevance [1171, 2125]. However, candiduria is a form of colonization, and thus its presence sharply increases the risk of invasive candidiasis. However, it alone is rarely diagnostic.
- Recovering Candida from a surgical wound or drainage or peritoneal fluid. Candida spp. are frequently recovered from these sites in surgical patients, however the isolation of Candida spp. from any of these sources is not diagnostic of localized infection [2129]. When Candida spp. are isolated from peritoneal fluid or drainage, the possibility of candidal peritonitis should be entertained.
- Recovering Candida from the sputum or in a (A):BAL specimen Candida spp. are normal commensals of the human mucous membranes and this includes the mouth. By extension, specimens from the airways may also be contaminated with Candida spp. It is particularly frequent to find Candida spp. in the respiratory tract of severely ill patients. However, clinically relevant candidal pneumonia is quite rare [1946] and the presence of Candida spp. in the sputum has only a loose association with pathologically documented pneumonia [653]. Consequently, the isolation of Candida spp. from the sputum, tracheal aspirates, and even BAL by protected specimen brush is not diagnostic by itself.
- Recovering Candida from the (A):CSF. The CSF should always be sterile, and a positive CSF culture could be due to CNS candidiasis. Positive CSF cultures are not uncommon as part of the picture of neonatal candidiasis. But, positive CSF cultures are quite uncommon in adults [2340] and the possibility of a contaminated specimen should be seriously entertained.
B. Serologic Data
Despite intense efforts and many years of hard work, no commercial test has yet been shown to routinely have clinical applicability. This is not due to a lack of test approaches which have at least reasonable sensitivity. Indeed, a number of systems have been shown to be able to detect the presence of invasive candidiasis. Rather, the key problems are that (a) as Candida spp. are normal commensals, all kits must use a relatively high cut-off (thus lowering their sensitivity) and (b) the pace of invasive candidiasis is usually so rapid that serodiagnostic tests don’t become positive until the diagnosis is also strongly suggested by other data.
Type of test Strengths & Weaknesses Reference ANTIBODIES Antibodies to Candida
- Many are against inadequately characterized antigenic extracts
- Non-standardized techniques
- Seen late in the course of disease
- Limited use in immunosuppressed patients
[305, 822, 898, 1246] ANTIGENS Mannan
- Sensitivity ~70%
- Short serum half-life
- Complicated measurement technique
[782, 1182] Undefined heat-labile glycoprotein antigen (CAND-TEC Candida Detection System from Ramco Laboratories, Houston, Texas)
- Simple
- Commercially available
- Sensitivity and specificity very variable but generally below 60%
- Testing multiple serum samples increases sensitivity
- Colonization produces positive results
[305, 682, 788, 824, 1634, 1795] b-D-Glucan
- Cross reactivity with multiple fungi
- Appears sensitive
[
