Overview
Oropharyngeal candidiasis (OPC) is the general term given to the oral infection caused by the yeast Candida. This condition is also often referred to informally as “thrush”. As we will discuss in detail in this section, OPC meets all the criteria to be considered an opportunistic infection. Whereas asymptomatic oral colonization may also occur, OPC implies the presence of signs and symptoms of infection.
Epidemiology
Candida spp. are part of the normal mouth flora in 25-50% of healthy individuals [1672]. Such carriage is referred to as asymptomatic colonization. Candida albicans is the most frequent colonizer (70-80%) but any of the non-albicans Candida spp. may be seen. Salivary flow, salivary pH, and glucose concentration are among the factors that influence the frequency of oral candidal colonization. Specifically, carriage rates are higher in:
- HIV-infected patients and patients with AIDS. In these patients, the rate of carriage is a function of the level of immunosuppression [2025]. Patients heavily treated with fluconazole more often carry non-albicans Candida spp. that are resistant to the azole antifungal agents [159, 1402, 1403, 1404, 1458].
- Hospitalized patients regardless of underlying disease [318].
- (A):Denture users [336]
- Diabetic patients [2224]
- Cancer patients [268]
OPC, on the other hand, goes beyond mere carriage to the presence of symptomatic infection. This transformation from asymptomatic colonization to symptomatic disease occurs most often in people in the extreme of their lives (neonates and the elderly), in patients with debilitating conditions, and in individuals receiving certain types of drug therapy [1672]:
Factors that promote development of symptomatic OPC
Treatment-related (iatrogenic) Disease-Related General Conditions
Local treatments
Inhaled steroids
Oropharyngeal irradiation [733]
Trauma (dentures) [2388]
Systemic treatments
Broad-spectrum antibiotics
Cytotoxic cancer1 therapy
Immunosuppressive therapy
Corticosteroids
Immunodeficiencies
HIV-AIDS [722]
CMC2 [1197]
Malignancies [268, 2470]
Endocrinopathies3
Adrenal dysfunction
Hypothyroidism
Age
Premature infants
Newborn healthy infants
Elderly individuals [1672]
Nutritional
Malnutrition
- The incidence of OPC among cancer patients varies depending on the type of neoplastic disease. An incidence of OPC was initially reported as ~25% in patients with solid tumors and up to 60% in patients with hematologic malignancies and/or following bone marrow transplantation. Due to the widespread use of prophylactic antifungal therapy in these groups, however, current rates of OPC have decreased significantly [268, 2470]. Patients subjected to radiotherapy for the treatment of oral and pharyngeal malignancies are also frequently affected with OPC [733].
- Chronic mucocutaneous candidiasis
- The many endocrinopathies of chronic mucocutaneous candidiasis are all at least somewhat related to OPC.
- OPC most often appears in diabetics who have poorly controlled blood sugar levels [2300].
Clinical Manifestations
Although the most frequent and familiar form of OPC is pseudomembranous candidiasis (or thrush), there are two additional distinctive presentations of OPC [2012]. The general characteristics of the three main forms of OPC are shown in the table, with a more detailed discussion following:
| Type | Site(s) affected | Appearance | Symptoms |
|---|---|---|---|
| Pseudomembranous (“thrush”) | Buccal mucosa, tongue, palate, uvula | White thick plaques that, when removed, leave an erythematous bleeding surface | Varies according to the extent and severity but includes burning, pain, and taste changes |
| Erythematous or atrophic (includes denture-induced stomatitis) | Palate, tongue | Diffuse erythema | Soreness |
| Angular cheilitis | Angles of mouth | Cracking and inflammation of the corner of the mouth | Pain, soreness, and/or burning |
Pseudomembranous Oropharyngeal Candidiasis
This is the most frequently recognized presentation. It is commonly called thrush and presents with raised confluent white to creamy elevated patches on a hyperemic base. This can be better appreciated when plaques are removed and the base bleeds. The lesions can involve any part of the mouth.Patients affected with pseudomembranous OPC complain of mouth soreness or pain, burning tongue, taste changes, or dryness. Chronic oral discomfort associated with this form of candidiasis, especially in patients with AIDS, may impair the intake of adequate oral nutrition and contribute to weight loss and inanition. The presence of odynophagia (pain on swallowing) suggests extension of the process to the form known as Esophageal Candidiasis.
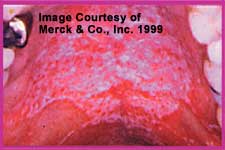
Mild Pseudomembranous OPC
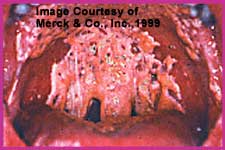
Severe Pseudomembranous OPC
-
Erythematous or Atrophic Oropharyngeal Candidiasis
Erythematous or atrophic oropharyngeal candidiasis presents with diffuse redness of the palate and the dorsum of the tongue. When the infection is principally on the tongue, the term candidal glossitis is used.Chronic atrophic candidiasis is associated with an indolent course. Some patients have no symptoms, whereas others complain of a metallic taste or a local burning sensation. Denture wearers seem especially prone to this condition, in which case it is referred to as (A):denture-induced stomatitis or denture sore mouth.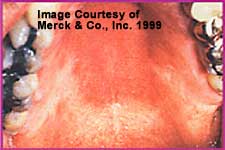
Erythematous candidiasis
-
Angular Cheilitis
This form of OPC has also been called “perleche.” It is characterized by inflammation of the angles of the mouth, with or without fissuring. Other causes of this picture include iron deficiency and Staphylococcus aureus infection. Patients are usually very symptomatic with local soreness, tenderness, pain or burning.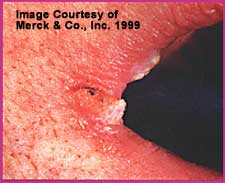
Angular cheilitis
Miscellaneous forms of OPC
Other forms of OPC have also been described. Candidal leukoplakia has been reported as a condition in which Candida are cultured from leukoplakic lesions [1255, 2322]. However, as the species composition of the mycoflora of these leukoplakic lesions does not differ from the species cultured from normal mucosa [1672, 1929], it is unclear whether Candida are playing a role. Thus, we do not currently list this as a separate form of OPC. In addition, there is a single report of an entity described as candidal glossitis [470], but this does not appear to represent a distinctive major entity.
Therapies
The many different therapeutic options available for treatment of oropharyngeal candidiasis are shown in the table. Topical therapy usually suffices for milder forms of the disease. However, extensive disease, disease in patients with immunosuppression (most notably, disease in HIV/AIDS patients), and disease in which there are symptoms that suggest esophageal involvement (e.g., pain on swallowing) are best treated with systemic therapy. Prolonged suppressive therapy may be required if the immunosuppressive condition does not remit.
| Drug | Dosage | Comments | References |
|---|---|---|---|
| Nystatin | Pastilles or lozenges: 200,000U qid x 7-14 daysSuspension: 500,000 Units by swish & swallow qid x 7-14 days | It has an unpleasant taste and may cause nausea and GI disturbance. Vaginal tablets in combination with unsweetened mints or chewing gum are better tolerated [899]. | [747, 1132, 1824] |
| Clotrimazole | Suck on 1 troche 5x day x 7-14 days | It is more palatable than nystatin but contains dextrose which may promote dental caries [899] | [1567, 1824, 2091] |
| Fluconazole | 100 mg/d x 7-14 days (a loading dose of 200mg has been recommended for immunosuppressed patients and/or severe OPC) | Fluconazole is superior to nystatin, clotrimazole, and ketoconazole [543]. High doses (up to 800mg/day) can be used in difficult cases. Success has been obtained even in cases of in vitro resistance [1900]. | [543, 747, 1035, 1223, 1824, 1825, 2025] |
| Itraconazole | Suspension: 200 mg (20 ml) qd by swish & swallow without food x 7-14 daysCapsules: 200 mg/day (taken with food) x 2-4 weeks | The capsules have limited bioavailability and their absorption is improved if they are taken with a fatty meal. The efficacy of the capsules is thought about equal to that of ketoconazole [257]. The solution has only been tested among HIV patients, but is much better absorbed and has shown an efficacy equivalent to fluconazole [895, 1788]. | [171, 539, 895] |
| Ketoconazole | 200-400 mg/day x 7-14 days | This agent has limited bioavailability, requires an acidic environment for best absorption, and causes liver toxicity. As it is less efficacious than fluconazole and itraconazole suspension, it is less frequently used. | [539, 1283] |
Difficult Clinical Situations
Oropharyngeal Candidiasis (OPC) in patients with HIV-AIDS
Early in the HIV epidemic, OPC was identified as not only an immunologic marker of HIV infection but also of rapid progression to (A):AIDS [1214]. Oropharyngeal candidiasis occurs in about one third to one half of HIV-seropositive persons and in up to 90% of patients with AIDS, making it the most frequent opportunistic infection in AIDS [722, 2025].
Symptomatic OPC is rare until the CD4+ T-cells fall to < 500 cells/mm3. As AIDS progresses, either because of lack of adequate therapy or resistance to antiretroviral therapy, the severity and permanent nature of AIDS-associated immunosuppression make this infection particularly difficult to permanently eradicate. The natural history in patients with advanced HIV infection is one of remission and then relapse.
Prophylaxis (or, really, chronic suppressive therapy) with azole antifungals to prevent OPC among HIV-infected individuals has been extensively assessed [864, 1444, 2162]. Such therapy is effective, but this form of long-term treatment has created the perfect setting for emergence of resistance [159, 1402, 1403, 1404, 1458]. Candida spp. resistant to antifungal agents become common after several years of therapy [1744, 1912].
Importantly, a remarkable reduction of the rate of all opportunistic infections, including OPC, was seen upon the introduction of combination therapy and particularly, highly active antiretroviral therapy (HAART) [1457, 1707, 2499]. Indeed, resolution of OPC solely in response to initiation of HAART has been noted. Thus, the observation of OPC in an AIDS patient in the modern era implies a need for both better antiretroviral therapy and for initiation of antifungal therapy.
Continuous vs. Intermittent therapy of Oropharyngeal candidiasis in AIDS patients
In general, routine prophylaxis of mucosal candidiasis, particularly OPC, has been discouraged [79, 103, 1912]. Three reasons exist for which the United States Public Health Service (USPHS) and the Infectious Disease Society of America (IDSA) have made this recommendation. Two of them have remained unchanged during the entire HIV epidemic. First, OPC is a condition associated with a very low mortality. Second, OPC usually has a very good response to standard therapy.
The third reason is the all-too-real possibility that prolonged therapy will induce antifungal resistance [1912]. This issue has been instead the subject of intense evaluation. Multiple observations have demonstrated that this event is frequent [159, 1402, 1403, 1404, 1458]. On the other hand, it has also been shown that antifungal agents can reduce the rate of relapse and colonization [1834, 2060]. This theoretical quandry has had, in most instances, a relatively practical solution–frequent relapses were treated with frequent therapy, and at some point frequent therapy becomes (nearly) continuous in any given patient.
The publication of a prospective randomized study on this topic provides a helpful perspective. Revankar et al. compared the use of continuous and intermittent suppressive therapy in patients with frequent and recurrent episodes of candidal esophagitis [1901]. A reduction in the rate of both relapses and colonization was seen with both regimens. While microbiologic resistance did develop in some patients, therapeutic responses generally remained excellent. The key difference between this study and early reports was the usage of effective antiretroviral therapy. The fact that many of the patients benefit to at least some extent may explain these results.
In part as a consequence of this work, the 1999 USPHS/IDSA Guidelines for the Prevention of Opportunistic Infections in Persons infected with HIV recommends considering the use of an oral azole (fluconazole or itraconazole solution) when “recurrences are frequent or severe” [80]. But, it should also be kept in mind that the best therapy for OPC is not an antifungal agent–every effort to provide an effective antiretroviral regimen should be made as this is the only therapy that provides true and long-lasting relief.
Denture-Related OPC
Dentures permit the accumulation of yeast between mucosal surfaces and the prosthesis. Local factors like trauma, maceration and endogenous epithelial changes related to the prosthesis (atrophy, hyperplasia, dysplasia) affect the barrier mechanism of the mucosa [336]. As a consequence, denture wearers often complain of sore and irritated gums. Although bacterial infection, mechanical irritation, and allergic reaction have been proposed as possible causes, infection with Candida spp. has often been implicated. This syndrome is referred to as candidal stomatitis, and rates as high as 65% have been reported. [336].
Effective therapy requires treatment of both the mouth and the denture. Elimination of fungal colonization of the denture may be difficult. Regular and extensive cleaning of the denture is helpful [2388]. Five-minute irradiation of the denture (immersed in water) in a 60 Hz microwave oven is very successful to eradicate Candida from dentures. However, modification of denture hardness may occur when using this method [600]. Regular cleansing and soaking of dentures in 0.25% chlorhexidine in combination with an antifungal regimen is an alternative strategy that has been described [1007]. Consistent usage of topical antiseptics such as Listerine and Peridex may also be useful in prevention of recurrence [1507].
