Diagnosing Fungal Infections Using Histopathology
Histopathology remains one of the major tools of diagnosis in mycology. The major advantages of histopathology are speed, low-cost, and the ability to provide a presumptive identification of the infecting fungus, as well as demonstrating the tissue reaction.
However, unless special techniques such as immunofluorescence are used, or the infecting fungus possesses unique structures such as spherules, definitive identification of the etiologic agent by histopathology is difficult. Nevertheless, it usually provides essential information before the fungus can be isolated in a mycology laboratory. Furthermore, histopathology is the only way to diagnose infections caused by Loboa loboi or Rhinosporidium seeberi, as cultivation techniques have to date been unsuccessful.
In this section, we provide information about the commonly used histopathologic staining methods and the usual appearances of the frequently encountered fungi in infected tissues [415, 602, 654, 1173, 2041, 2064]. For histopathologic appearances of fungi other than those mentioned below or for more detailed information about fungi noted below, please refer to the related page for that specific fungus. The microscopic appearance in tissue of each of the fungi is also shown on the related page.
Stains Used in Histopathology
A number of histologic stains are available that are routinely used to visualize fungi in tissue sections. While some of these are special fungal stains, others are of more general use but still help to observe the tissue reactions and/or the infecting fungus. The reactions involved and comments of particular interest for some of these stains are summarized in the TABLE.
Gomori methenamine silver (GMS), Gridley’s fungus (GF), and periodic acid-Schiff (PAS) are special for and very efficient to visualize the fungi. Among these three, GMS is more advantageous since it stains old and nonviable fungal elements more efficiently than the other two. Hematoxylin and eosin (H&E) stain, on the other hand, is very useful to visualize the host’s response but is not a special fungal stain. It does not stain most of the fungi, except the Aspergillus spp. and the zygomycetes. Thus, a combination of GMS and H&E is usually employed to visualize both the tissue reaction and the infecting fungus.
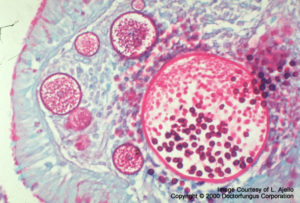
Rhinosporidium seeber
Mucin stains, like Mayer’s mucicarmine, Southgate’s mucicarmine and Alcian blue, stain the mucopolysaccharide capsule of Cryptococcus neoformans.
However, they are not specific for this particular fungus. Blastomyces dermatitidis and Rhinosporidium seeberi may also be stained with the mucin stains.
Histopathologic Features of the Most Commonly Encountered Fungi
Achieving a successful histopathologic diagnosis begins with selection of the tissue sample to be examined. While some fungi are more readily located at the periphery of the infected lesion, others are more prominent at the center. Then comes the use of appropriate stains and examination by experienced eyes. The major growth forms of the fungi that help in histopathologic diagnosis are the yeast cells, hyphae, pseudohyphae, arthroconidia, chlamydoconidia, and spherules. The characteristics of each of these (shape, size, location, color…) help in identification of the fungus. Tissue components such as Russell bodies, karyorrhectic debris, calcified bodies, elastic fibers, and small blood vessels may resemble fungi. Careful examination by experienced specialists significantly reduces such an overdiagnosis.
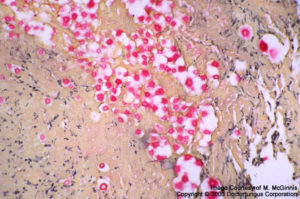
Cryptococcus neoformans
Candida: Budding yeast cells (blastoconidia), pseudohyphae and septate hyphae are observed.
Cryptococcus neoformans: Pleomorphic yeast-like cells and formation of narrow-based buds are typical. The encapsulated strains have capsular material detected with mucin stain.
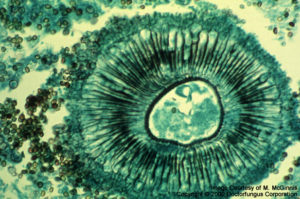
Aspergillus niger
Aspergillus: Hyaline, septate, dichotomously branched hyphae of uniform diameter are observed. In cavitary lesions, conidial heads are occasionally observed. Purulent, necrotizing inflammation is present. Calcium oxalate crystals, probably originating from fungal oxalic acid, are present near hyphae in necrotic tissue, if the infecting species is Aspergillus niger.
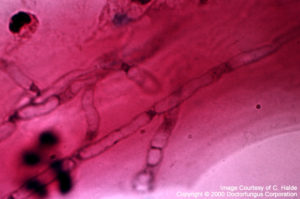
Fusarium
Fusarium and Pseudallescheria: Hyaline, septate, randomly branched hyphae are observed. The hyphal contours are regular.
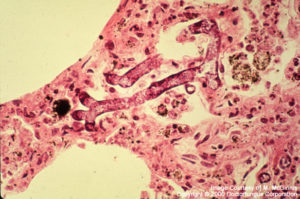
Rhizopus
Mucor, Rhizopus and other fungi of the Order Zygomycetes: Broad, thin-walled, hyaline, often aseptate or sparsely septate hyphae are observed. The contours of the hyphae are typically non-parallel and branches are irregular. Invasion of blood vessels is noteworthy. Pyogenic inflammation, abscess formation and suppurative necrosis are observed.
Microsporum, Epidermophyton, and Trichophyton: These fungi, the dermatophytes, produce septate, branched hyphae that break into chains of arthroconidia.
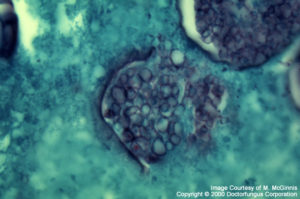
Coccidioides immitis
Coccidioides immitis: Spherule containing endospores is the typical structure. The transition form of C. immitis producing septate hyphae that develop into arthroconidia, may be observed in necrotic nodules and misdiagnosed as one of the fungi in hyphomycetes group, when spherules are not yet evident.
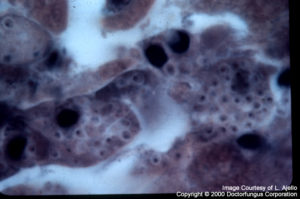
Histoplasma capsulatum
Histoplasma capsulatum: Intracellular budding yeast cells are typical. It should be differentiated from the other intracellular dimorphic fungus, Penicillium marneffei. The yeast cells of P. marneffei divide by fission, but not by budding. Histoplasma capsulatum predominates at the central portion of the lesions. Thus, the selection of the tissue section to be excised for histopathologic and mycologic examination is of particular significance.
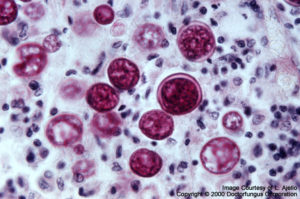
Blastomyces dermatitidis
Blastomyces dermatitidis: Thick-walled yeast-like cells and single broad-based buds are typical. In contrast to Histoplasma capsulatum, Blastomyces dermatitidis is located more at the periphery of the infected lesion, rather than the center.
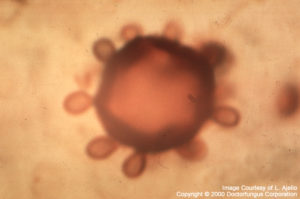
Paracoccidioides brasiliensis
Paracoccidioides brasiliensis: Yeast-like cells with multiple buds are typical. The buds are attached to the mother cell with narrow necks. The appearance resembles a “steering-wheel”.
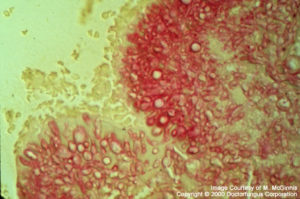
Madurella
Fungi causing eumycotic mycetoma (Madurella, Acremonium, Pseudallescheria, Exophiala, Curvularia, Aspergillus, and others): Granule formation is typical. The granules are composed of hyaline or phaeoid (depending on the fungus involved), septate, branching hyphae. Chlamydoconidia may be formed.
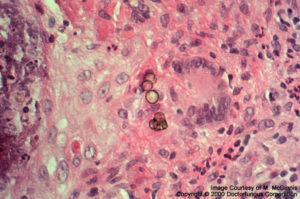
Fonsecaea
Fungi causing chromoblastomycosis (Fonsecaea, Phialophora, Cladophialophora, and others): Spherical or polyhedral, dark brown, thick-walled sclerotic bodies (muriform cells) are typical. Phaeoid hyphae may also be observed.
TABLE
| Stain | Reaction | Comments |
|---|---|---|
| Fontana-Masson | Chromaffin reaction probably oxidizes melanin or melanin precursor as it reduces silver. Cell walls black. Background pale pink. | Useful for staining cell walls of dematiaceous fungi when the nature of their cell walls is not evident, and useful for Cryptococcus neoformans. Natural color of cell walls masked. |
| Grocott’s modification of Gomori’s methenamine silver method | Chromic acid oxidizes cell-wall polysaccharides to aldehydes. Aldehyde products reduce methenamine silver nitrate to metallic silver. Cell walls brown to black. Background pale green. | Enhances visualization of fungi and their morphology. Natural color of cell walls masked. Erythrocytes and naked nuclei can mimic yeasts. Tissue response cannot be studied. Best contrast of the special fungal stains. |
| Gridley’s stain | Chromic acid oxidizes adjacent hydroxyl groups of cell-wall polysaccharides to aldehydes. Aldehyde groups combine with Schiff reagent. Aldehyde fuchsin occupies uninvolved linkages of the Schiff reagent. Cell walls purple to magenta, yeasts rose to purple, and capsules deep purple. Background yellow. | Enhances visualization of fungi and their morphology. Natural color of cell walls masked. Tissue response cannot be studied. |
| Hematoxylin and eosin | Hematein-mordant stains DNA in the nucleus and nuclear proteins. Nuclei blue, cartilage and calcium deposits dark blue, cytoplasm and other components shades of red, and erythrocytes bright red. | Visualizes host tissue response to the fungus. Aspergillus spp. and Zygomycetes stain well. Difficult to distinguish some types of fungi, as well as locate fungi in tissue if they are present in small numbers. |
| Immunofluorescence | Direct technique: fluoresceinlabeled antibody reacts with fungal antigens in cell wall. Indirect technique: unlabeled antibody complexes with fungal antigens. Fluorescein-labeled conjugate reacts with globulins attached to fungal antigens. Cell walls yellow-green. | Some use the immunoperoxidase technique. Specific and highly sensitive. Can be used to detect and measure antibodies. |
